Environmental Engineering Reference
In-Depth Information
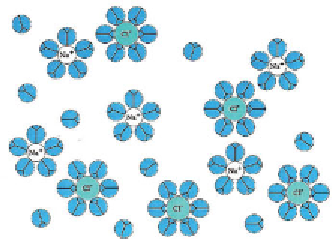
Figure 3.8
Schematic representation of NaCl dissolved in water (after Hill et al, 1997).
Water molecules (in blue), with positive poles adjacent to Cl- anions and negative Poles
adjacent to Na+ cations.
Si
3
O
9
Si
4
O
12
c
d
Si
2
O
7
b
pH
SiO
4
Scale of
Ångström units
Si
6
O
15
0
1
2
3
e
a
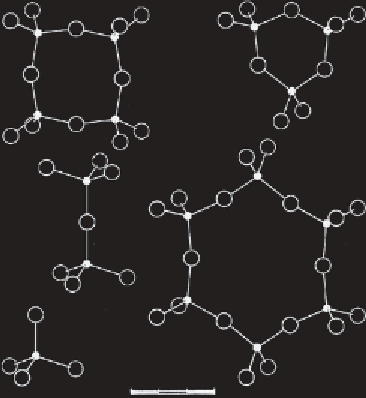
Figure 3.9
Silicate tetrahedra linkage (after Berry and Mason, 1959). a) Independent
tetrahedra; b) Double tetrahedra; c - e) Ring structures.
These silica tetrahedra are the building blocks of silicate minerals, much
like methane tetrahedra are building blocks of organic molecules (see fig-
ure 3.9). Unlike hydrocarbons, most of which are either gases or liquids, at
room temperature, however, silicates are all solid crystals.
In addition to the simple silicate tetrahedra, silicates also form single
and double chains, as well as sheet structures and three-dimensional net-
works (see figure 3.9). The common feature, of silicate crystal lattices is that
they all consist of cations surrounded by anions and/or silicate radicals.















Search WWH ::

Custom Search