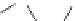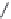Biology Reference
In-Depth Information
COO
−
COO
−
COO
−
COO
−
COO
−
COO
−
COO
−
COO
−
COO
−
COO
−
N
N
N
N
N
OO
OMe
OO
diazo-2
diazo-3
diazo-4
Me
O
O
O
O
N
+
N
+
N
+
+
N
N
−
N
−
−
N
N
−
Fig. 4
Structures of the diazo series of photolabile chelators, which take up calcium on exposure to
light.
Ca
2+
CO
−
−
O
2
C
CO
−
−
O
2
C
CO
−
−
O
2
C
−
O
2
C
CO
−
CO
−
CO
−
−
O
2
C
−
O
2
C
N
N
N
N
N
N
H
2
O
h
n
O
O
O
O
O
O
CH
3
CH
3
HC
CH
3
H
2
C
H
+
O
+
+
N
2
CH
C
CO
−
O
N
2
Photolyzed diazo-2
(high Ca
2+
affinity)
Diazo-2
(low Ca
2+
affinity)
Fig. 5
Reaction scheme for the photolysis of diazo-2.
ionic strength). The absorbance maximum of the photosensitive group is
22,200 M
l
cm
1
at 370 nm, and drops to negligible levels at this wavelength
after photolysis. A small remaining absorbance reflects formation of a side product
of unenhanced a
Y
nity and unchanged molar extinction coe
Y
cient in 10% of the
ective photon absorption. This ''inactivated'' diazo still binds Ca
2
þ
(with some reduction in absorbance), but is incapable of further photolysis. The
Ca
2
þ
-bound form of diazo-2 has about one-tenth the absorbance of the free form,
dropping to negligible levels after photolysis, with quantum e
instances of e
V
ciency of 0.057 and
a time constant of 134
m
s. Binding of Ca
2
þ
to photolyzed diazo-2 is fast, with an
on-rate of 8
Y
10
8
M
1
s
1
.Mg
2
þ
binding is weak, dropping from 5.5 to 3.4 mM
after photolysis, and pH interference is small with this class of compound.
One limitation of diazo-2 is that the unphotolyzed chelator has su
cient Ca
2
þ
Y
a
nity that its incorporation into cytoplasm is likely to reduce resting levels to
some degree, and certainly will have some e
Y
ect on [Ca
2
þ
]
i
rises that occur
physiologically. To obviate this problem, diazo-4 was developed with two
V