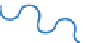Biology Reference
In-Depth Information
A
Internal conversion, vibration state
(loss of energy) (ps)
High energy
excited states
Emission
(ns)
Absorption
(excitation)
(fs)
Emission light
(longer wavelength)
Excitation light
Low energy
ground state
B
Stokes shift
Emission
Absorption
(excitation)
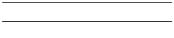
Wavelength
Fig. 2
(A) A Jablonski diagram showing the energy states of a given fluorophore, including the
process of absorption and emission of longer wavelength light upon excitation. The duration of each
state is also indicated. (B) The absorption and emission spectra of a given fluorophore including the
Stoke's shift due to emission of a longer wavelength photon upon excitation of the fluorophore.
V. Total Internal Reflection Fluorescence Microscopy
Confocal microscopy coupled to TIRF provides a very thin optical section
of fluorescence excitation that allows imaging with low background noise and
minimal out-of-focus fluorescence. This is because total internal reflection can
only occur when the excitation light beam in a medium of high refractive index
reaches an interface of a medium with a lower refractive index at an angle of
incidence that is greater than the specific critical angle
y
. When the light is
totally internally reflected, none of it penetrates the medium with the lower
refractive index, and ideally, there is no net energy flux escaping the glass.