Biology Reference
In-Depth Information
A
B
1.2
−
BAPTA
+
BAPTA
1.0
0.8
0.6
0.4
0.2
0.0
0
10
20
30
0
10
20
Time (min)
30
Time (min)
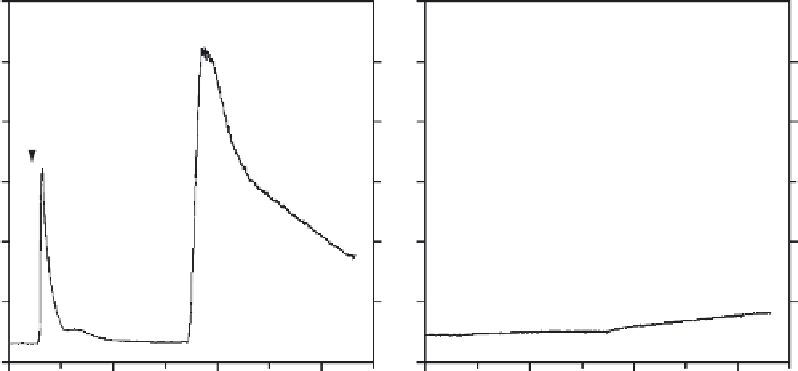
Fig. 9
Bu
V
ering action of intracellular BAPTA on changes in [Ca
2
þ
]
i
. (A) Changes in [Ca
2
þ
]
i
of a
REF52 cell treated with 1
m
M bradykinin and then 1
m
MBr-A23187. (B) Changes in [Ca
2
þ
]
i
of a REF52
cell, preloaded with BAPTA, in response to the same treatments as in (A). Cells were loaded with 1
m
M
Fura-2 AM in Pluronic dispersion for 85 min at 25
C. For (B), 20
m
M BAPTA AMwas also present in
the incubation medium. Experiments were done in HBSS.
Indeed, even the massive rise resulting from a combination of Ca
2
þ
influx and
discharging of intracellular calcium stores mediated by Br-A23187 is suppressed
substantially by the bu
V
ering action of BAPTA.
2. Possible Controls for the Use of BAPTA
Similar to EGTA, BAPTA is a chelator not only for Ca
2
þ
but also for other
multivalent metal cations. Thus, one may wish to ensure that any inhibitory e
V
ect
observed when using BAPTA is caused strictly by the ability of BAPTA to bu
er
Ca
2
þ
, and not because it is scavenging other biochemically important metal ions
such as Zn
2
þ
. The reagent used to control for heavy metal scavenging by BAPTA is
TPEN (N,N,N
0
,N
0
-tetrakis(2-pyridylmethyl)ethylenediamine) (
Fig. 10
), a mem-
brane-permeant metal ion chelator that shows a marked preference for binding
heavy metal cations over Ca
2
þ
(
Anderegg et al., 1977
). Whereas the K
d
(Ca
2
þ
)of
TPEN is 40
m
M(
Arslan et al., 1985
), K
d
(Zn
2
þ
) is 2.6
V
10
16
M(
Anderegg and
Wenk, 1967
). This enormous selectivity for binding heavy metal ions over Ca
2
þ
enables TPEN to scavenge heavy metal ions very e
V
ectively, even in the presence of