Environmental Engineering Reference
In-Depth Information
solution of technical
problems
100
80
application of innovative materials and
new production technology
60
40
introduction into market
20
0
2010
prototype
2015
prototype
2020
first small quantity
production
2025
quantity
production
years
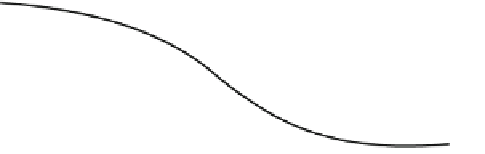
Fig. 17.8
Required reduction of expenses in fuel cell technology
The electric car as a storage system is still a research project for the regulation
and storage of electricity in a future grid, but it seems to be very meaningful.
17.1.2.5 Fuel Cell Technology
Fuel cells function similar to batteries and produce electricity from chemical
reactions of H
2
and O
2
by combustion without flame at lower temperatures in the
presence of catalysts. They replace large, heavy batteries, and run with a high
efficiency and low emissions. Recent models are usable from a few Watts to a few
Megawatts.
However, the price of fuel cell technology must be greatly decreased before it
become popular; see Fig.
17.8
[
20
].
In solid oxide fuel cells, the temperature of operation decreases from 800Cto
650C, i.e., from 1,472F to 1,202F. The technology uses a high power density
and is of optimal durability. The oxide layer could be sprayed by an automated
process which could be a path towards cost-effective mass production [
21
].
Solid Acid Fuel Cells operate at low temperatures, have a performance of
250 W (0.34 HP), and use diesel fuel for the hydrolysis. One predicts they are the
best solution for the future [
22
].
Currently, there is no enough data on the life span and durability of fuel cells in
regular use. The most important application of fuel cells is the production of
energy in spacecraft and in submarines. This is still a very small field. Profitable
mass production seems to only be realistic in the future.
17.1.2.6 Hydrogen in Fuel Cells
Hydrogen can be burned like gasoline inside the engine or used in fuel cells to
generate power. A hydrogen fuel cell produces current made from hydrogen and











Search WWH ::

Custom Search