Environmental Engineering Reference
In-Depth Information
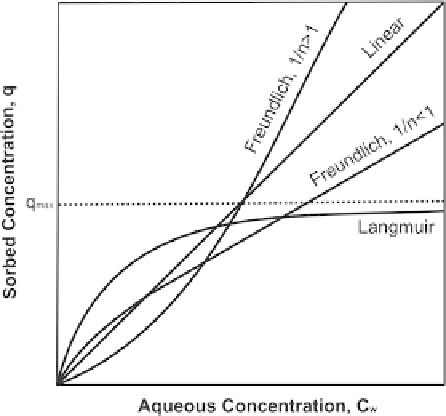
Fig. 19.5
Schematic
illustration of sorption
isotherms
However, especially if adsorption prevails, the concentration in soil usually
depends non-linearly on the concentration in the groundwater (non-linear sorption).
For non-linear isotherms (Fig.
19.5
) the Freundlich and Langmuir models are often
used. The Freundlich isotherm can be written as:
K
Fr
C
1
/
n
C
s
=
(19.13)
w
where
K
Fr
[(MM
−
1
)/(M L
−
3
)
1/n
] is the Freundlich sorption coefficient and
1/n
is an
empirical exponent. The Freundlich isotherm reduces to the linear model, analogous
to Eq. (
19.12
), if 1/
n
1.
The Langmuir model represents another non-linear isotherm which takes into
account a maximum sorption capacity:
=
K
L
C
s
,max
C
w
1
C
s
=
(19.14)
+
K
L
C
w
where
C
s
,max
[M M
−
1
] is the maximum concentration in the solid and
K
L
[L
3
M
−
1
]
is the Langmuir sorption coefficient. For
K
L
C
W
<< 1 the Langmuir isotherm predicts
a linear relationship analogous to Eq. (
19.12
).
In groundwater, sorption causes retardation of the advective and dispersive trans-
port of dissolved contaminants. Sorption processes typically influence the time it
takes for a contaminant to travel a certain distance and the overall plume to reach
steady-state. After steady state is achieved, the sorption capacity generally does not
influence the length of the plume (Liedl et al.
2005
). However, sorption processes
play an important role under transient flow and transport conditions (Cirpka
2005
;
Prommer et al.
2002
).
Search WWH ::

Custom Search