Environmental Engineering Reference
In-Depth Information
(a)
R-COO
-
+ Cd
2+
⇔
R-COOCd
+
R-COO
-
(b)
R-COO
+ Cu
2+
⇔
Cu
R-O
-
R-O
Fig. 16.1
Cadmium complexed to one deprotonated carboxylate group (
a
), and copper complexed
to one carboxylate and one neighbouring phenolate group, as an illustration of the formation of
chelate complexes
matter (McBride
1994
), with copper in particular able to form a strong bound.
Overall, total metal-binding concentrations to organic matter decrease in the order
copper > nickel > lead > cobalt > cadmium > calcium > zinc > manganese > mag-
nesium (McBride
1994
). Unfortunately, there is currently no systematic explanation
for this. The difficulty in determining the reason for this variation is probably due to
the wide variety of functional groups present on organic matter. In the “real” world,
the strength of metal binding is to a considerable extent dependent on competition
for binding sites with other commonly occurring cations, such as H
+
and macro-ions
like Ca
2+
,Mg
2+
,Na
+
, et cetera.
The redox conditions in soil will also impact bioavailability of metals. In gen-
eral terms, for elements that can exist in more than one oxidation state the lower
oxidation state ions are more soluble. So under more reducing conditions the con-
centration in the pore water often increases. If soils are water-logged and become
anaerobic, oxyhydroxides of Fe and Mn become unstable and dissolve. Any sorbed
ions are released and there is an initial increase in bioavailability. Over time,
bioavailability can decrease as pore water is leached from the upper soils. In
waterlogged soils, however, leaching rates are relatively slow. Release of ions into
the pore water initiated by waterlogging is partially offset by the precipitation of
sulphides, which can reduce metal availability. Indeed, some in situ remediation
techniques rely on generating reducing conditions to render metals unavailable.
Finally, there is increasing evidence that speciation of metals governs their
bioavailability. Indeed, models such as the
Biotic ligand model
(Section
16.4.2.1
)
rely on this phenomenon (Allen et al.
2008
; Arnold et al.
2007
;DiToroetal.
2001
;
Lock et al.
2007
; Steenbergen et al.
2005
; Thakali et al.
2006a, b
; Van Gestel and
Koolhaas
2004
). Generally, metals present as free ions or as simple, relatively small
inorganic complexes are viewed as being available, whilst larger, often organic,
complexes are not. In general, the higher the ionic strength of the pore water, the
less free ions are present, and therefore the lower the bioavailability of that metal.
16.3.2 Organic Contaminants
The factors affecting the bioavailability of organic contaminants are fundamen-
tally the same as those for metals, but the magnitude of effects varies. To assess
the bioavailability of organic contaminants it is also necessary to determine the

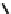


Search WWH ::

Custom Search