Environmental Engineering Reference
In-Depth Information
100
100
10
10
1
1
-NTA
+NTA
0.1
0.1
-NTA
+NTA
0.01
0.01
1E-10
1E-09
1E-08
1E-07
1E-06
1E-05
1E-10
1E-09
1E-08
1E-07
1E-06
1E-05
Cd
sol
(M)
Cd
2+
(M)
Fig. 8.3
Speciation affects shoot cadmium concentration in
Thlaspi caerulescens
grown in nutri-
ent solution. Left: the addition of the Cd
2+
chelator nitrilotriacetic acid (NTA) decreases cadmium
uptake when compared at equal soluble cadmium concentration. These data illustrate that the free
ion is preferred compared to complexed cadmium. Right: same uptake data as left but plotted to
the predicted free ion activity in solution, illustrating that the chelator addition increases the avail-
ability of the free metal ion, i.e. the Free Ion Activity uptake Model (FIAM) is not fully valid.
Note that the curves merge at large free ion activity suggesting that the deviation of FIAM at lower
activities are not due to partial uptake of Cd-NTA but due to the mechanism of buffering Cd
2+
in
the unstirred layer adjacent to roots (see text for argumentation; unpublished data from F. Degryse)
root uptake rate to the free metal ion activity in solution (Parker et al.
1995
). For
that reason, experimental methods have been developed to measure the free metal
ion activity in soil. The FIAM has been contested, however, and it is now clear
that the free metal ion is generally the preferred species, but that the complexed
(and adsorbed) species contribute to availability as well, depending on timescales
considered. For example, it is observed that metal uptake increases in soil when
the concentration of a complexed metal increases at constant free metal activity
(e.g. Smolders and McLaughlin
1996a, b
). Such is also illustrated in Fig.
8.3b
that
presents the same data as in Fig.
8.3a
but now plotted in a free metal ion activity
basis. Here, it is shown that the complexed metals have an 'apparent availability' in
nutrient solution. Experimental evidence has now clarified that this apparent avail-
ability is related to the ability of a metal complex to buffer the free metal ion uptake
at the root surface (Degryse et al.
2006a
). In soils and even in stirred nutrient solu-
tion, there is a zone adjacent to roots where the rapid intake of the metal or metalloid
ion is not readily replenished by the flow of water to the roots. As a result, the free
ion activity decreases near the root surface (Fig.
8.4
). With increasing concentra-
tions of soluble complexes and at constant free ion activity, such depletions are less
pronounced and bioavailability of the metal is enhanced. Effectively, this means that
the apparent availability of the metal complex is not by direct uptake of the com-
plex, but a consequence of the lack of a fully mixed system in the root environment.
This concept now also explains why root exudates enhance both solubility as well
as bioavailability of the metals copper and zinc, despite the fact that the exudates
do not change the free metal ion activity in soil (Degryse et al.
2008
). There is also
evidence that intact metal complexes can be absorbed (Collins et al.
2001
, Tandy










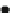






















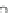



























Search WWH ::

Custom Search