Environmental Engineering Reference
In-Depth Information
ML
n±
Soil pore water
3
1
4
M
n+
M
n+
M
2
Mineral or
organic matter
MX
Fig. 8.1
Relationship between cationic metals in discrete mineral phases (MX), free cationic metal
ions in pore water (M
n+
), complexed metals in pore water (ML), metals sorbed to soil charged
surfaces, and metals occluded within minerals or organic matter in soil. Reaction represents
sorption/desorption reactions and is described by a sorption coefficient, reaction represents
precipitation/dissolution reactions and is described by a solubility product, reaction represents
a solution complexation reaction and is described by an association constant, and reaction is
generally termed an 'ageing' reaction whereby sorbed metals become less available with time
(McLaughlin
2001b
)
reactions, as well as precipitation/dissolution reactions (Fig.
8.1
). This obviously
depends on the degree of and type of soil contamination. Soils highly contaminated
by soluble metal sources are more likely to have distinguishable metal precipitates,
as are those soils contaminated by sparingly soluble materials. Sorption/desorption
processes are more likely to control metal partitioning in soils contaminated by
lower concentrations of soluble contaminants, or in soils contaminated by highly
soluble solid materials. The impact of both reactions combined is represented by
the
K
d
.
There have been numerous studies of metal partitioning in soils and how
K
d
values are affected by soil mineralogy, particle size, pH, salinity, redox status, metal
loading, etc. and the reader is referred to several review articles that summarise
these findings (Buchter et al.
1989
; Degryse et al.
2009
; McBride
1989
; Sauvé et al.
2000
). The ranges of
K
d
values found for various metals are shown in Table
8.2
.
The most important factor found to influence the dissolution of cationic metal
precipitates, and the release of cationic metals by negatively charged soil surfaces,
is soil pH. Hence higher concentrations of cationic metals are found in the pore
waters of acidic soils. Moreover, raising soil pH by liming will significantly reduce
cationic metal concentrations in soil pore waters. Soil pH is therefore a master vari-
able in controlling the distribution of cationic metals between the solid phase and
the pore water, and hence their availability to plants. Soil particle size and miner-
alogy also influence
K
d
values, with sandy soils or soils having low concentrations
of high surface area minerals having low
K
d
values. Sandy acidic soils are therefore
most at risk of having high concentrations of cationic metals in soil pore water, and
hence a risk of plant uptake of these contaminants. Due to the inverse relationship










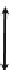



Search WWH ::

Custom Search