Environmental Engineering Reference
In-Depth Information
It can then be assumed that only a fraction of the inorganic contaminant will be
absorbed by the receptor (human or animal), this fraction is called the
bioavailable
fraction
. As oral exposure is being considered the term oral bioavailability is used.
When using a Risk Assessment model, it is therefore very important to understand
whether the underlying algorithm incorporates bioavailability in the oral exposure
pathway. This chapter focuses on oral bioavailability (OB) to humans.
7.1.1 Oral Bioavailability
Bioavailability can be defined as the fraction of an ingested contaminant that is
absorbed and reaches the systemic circulation where it may then cause adverse
effects on human health. Oral bioavailability can be assessed by comparing the
internal doses obtained after oral administration to that of intravenous adminis-
tration of the contaminant. The absolute oral availability is defined as the ratio of
the oral administration (AUC
PO
) to the intravenous administration (AUC
IV
). The
internal dose is often related to the area under the curve (AUC), using the rela-
tionship between time and plasmatic concentrations of the contaminant (Fig.
7.1
).
However, other end-points and methods of determining the oral bioavailability of
contaminants are commonly used and briefly discussed in Section
7.1.2
.
Oral bioavailability is of toxicological interest because the possible adverse
effects, caused by the contaminant, on the exposed human subject are related to
the internal dose. In order to depict the effect of soil properties on contaminant
availability, this concept can be divided into several steps;
accessibility
,
absorption
and
metabolism
.
The most commonly used definition of oral bioavailability is based upon a two-
step model with three compartments shown in Fig.
7.2
and Eq. (
7.1
):
F
Bacc
×
F
Abs
=
F
Bava
(7.1)
A slightly different definition (used by the Dutch National institute of pub-
lic health and the environment,
RIVM
) includes a third step with liver action
(metabolism and possibly bile excretion) on the contaminant as defined by Eq. (
7.2
):
Plasmatic concentrations
after intraveinous administration (IV)
c
Plasmatic concentrations
after oral administration (PO)
t
AUC
IV
AUC
PO
Fig. 7.1
Oral Bioavailability pharmako-kinetics definition









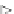















Search WWH ::

Custom Search