Environmental Engineering Reference
In-Depth Information
The theoretical options for endpoints in Human Health Risk Assessment are
almost inexhaustible, for instance, enzymatic activity, membrane potential, secre-
tion of a hormone, heart rate, or muscle contraction. Human data on reproduction
effects, neurological effects, organ toxicity, mutagenesis and carcinogenic effects
are available for several contaminants. However, these effect data are often char-
acterized by a poor experimental quality, in some cases, because the conditions
of exposure are not always known. Therefore, most Critical Exposure values are
derived from animal experiments. Examples of endpoints in animal experiments are
alteration of morphology, growth, mass, or life span.
For the purpose of deriving a limit value for exposure (a Critical Exposure value
as Toxicological Reference Value),
dose-response relationships
are derived for spe-
cific organisms. Such a dose-response curve is derived by exposing the organism
to a contaminant at a gradient of concentrations or exposures, often equally spaced
on a logarithmic scale (e.g., a concentration of 0.3, 1, 3, 10, 30, 100 mg/kg
dw
). The
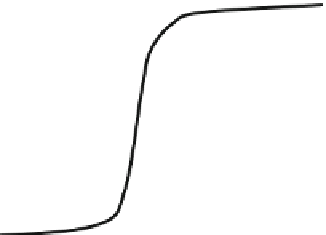
dose-response relationship often shows an S-curve. In Fig.
5.4
a hypothetical typical
S-shaped dose-response relationship is presented. Essential factors in a dose-
response curve are the no-effect range, the maximum effect range and, in between,
the range of increasing effect with increasing dose. Occasionally, dose-response
relationships are U-shaped, such as in case of
hormesis
, for example (
hormesis
is
Greek for 'stimulation', i.e., the biological effect at which a contaminant is harmful
at higher doses and low dose, but less harmful at intermediate dose; Calabrese and
Baldwin (
2001
)).
The most common effect measurement in animal experiments is the
No-observed-adverse-effect-level
(NOAEL), in other words, the highest experi-
mental dose or concentration at which no adverse effects are shown. In fact the
NOAEL is the best available alternative for the actual effect measurement, that
is, the
No-adverse-effect-level
(NAEL). When no NOAEL is available, a
Lowest-
observed-adverse-effect-level
(LOAEL), in other words, the lowest experimental
dose in which the adverse effects are shown, could be used as representative of
the NAEL. Since the NAEL is equal to or higher than the NOAEL and always lower
Maximum
effect range
No-effect
range
Range of increasing
effect with
increasing dose
Fig. 5.4
A typical
dose-response relationship
(S-curve;
solid line
)
Dose (Concentration (mg.kg
-1
dw
) or exposure (g.kg
-1
bw
.d
-1
))



Search WWH ::

Custom Search