Environmental Engineering Reference
In-Depth Information
respectively;
s
- border tension between the hydrocarbon-phase and the reser-
voir water;
H
-depth of reservoir;
g
-acceleration of free fall;
r
-densityof reser-
voir water;
r
- densityof the hydrocarbonphase.
From the known Arrhenius equation the coefficient of the reaction velocity of
degradation is equal to
Ae
−
E RT
e=
(5.29)
where,
E
-the activation energyof degradation;
R
- Universal gas constant;T-
T
- Ab-
solute temperatureof the system.
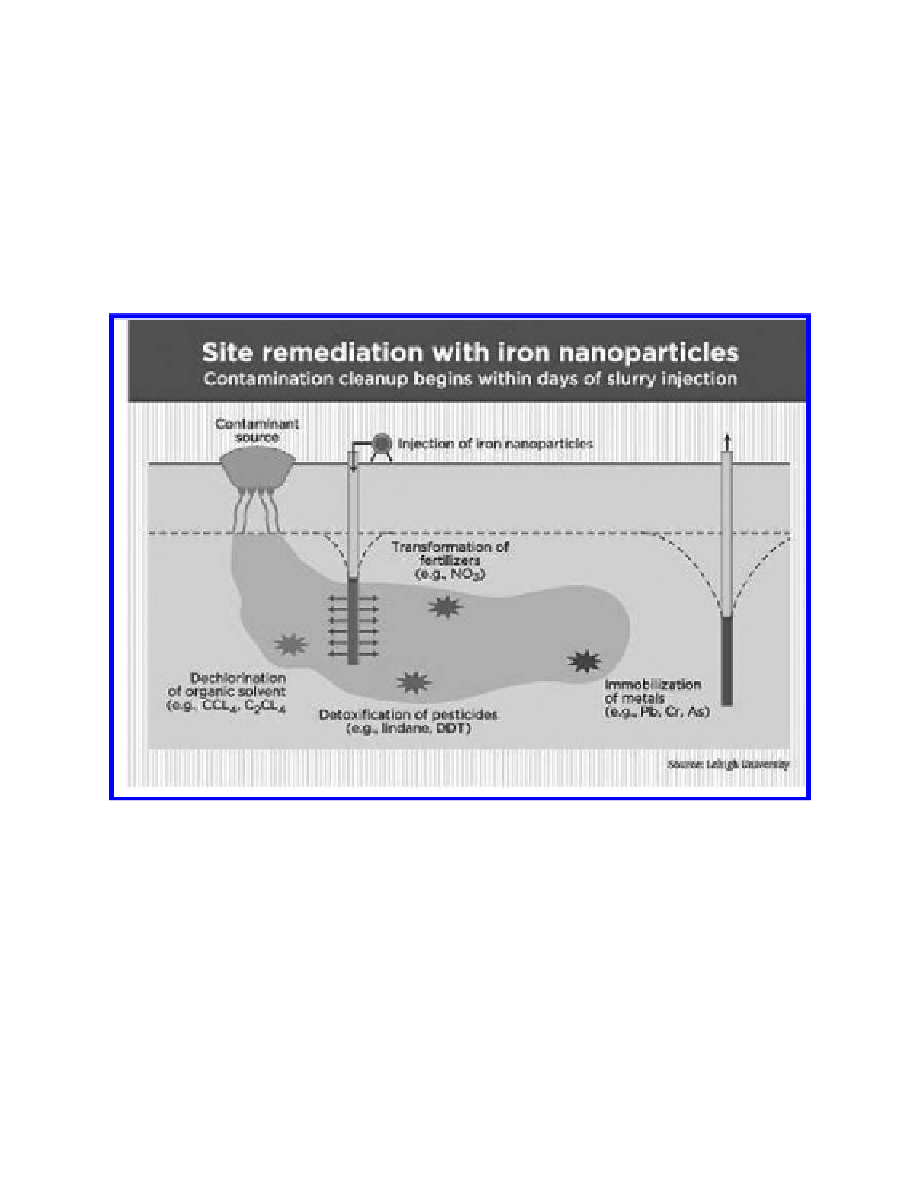
FIGURE 5.2
Cleaning the area with iron nanoparticles.
5.4 PROPERTIES OF ALUMINUM
Aluminum powder reacts with water at 50°C with an allocation of hydrogen. It
reacts vigorously in exothermic reactions with oxygen-containing liquids with
halogenated organic compounds and other oxidants.
Aluminum is relatively non-toxic, relatively inexpensive, widely distributed
in nature and in large quantities produced in industry by the electrolysis.
Aluminumin the form ofnanopowderhaslowered the ability to reaction at
room temperaturedue to the presenceof denseoxide-hydroxide shell, which is an
electric doublelayer.
Search WWH ::

Custom Search