Environmental Engineering Reference
In-Depth Information
n
n
∑
=
M
=
m
∑
=
obvious that
and
g
=
1
. The volume fraction is
, where
i
i
i
1
i
1
v
-partial volume ofcomponentmixtures.
Similarly, we have
Depending on specificity of tasks the gas constant of the mixture may be de-
termined as follows:
If we know the gas constant
, the seeming molecular weight of the mixture
is equal to:
The pressure of the gas mixture
p
is equal to the sum of the partial pressures
of individual components in the mixture
p
i
:
i
1
n
p
p
(2.5)
i
i
1
Partial pressure
p
i
-pressure that has gas, if it is one at the same temperature
fills the whole volume of the mixture .
With various methods of setting the gas mixture partial pressures
pg
m
m
p
=
pr
p
=
pr
;
p
=
i
cm
;
(2.6)
i
i
i
11
i
From the Eq. (2.6) we see that for the calculation of the partial pressures
p
i
nec-
essary to know the pressure of the gas mixture, the volume or mass fraction
i
of
the gas component, as well as the molecular weight of the gas mixture m and the
molecular weight of
i
of gas mi.
i
.
The relationship between mass and volume fractions are written as follows:
m
r
v
R
m
g
=
i
=
i
i
=
см
r
=
i
r
i
i
i
m
r
v
R
m
см
см
см
i
см
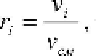
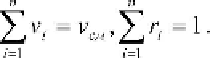


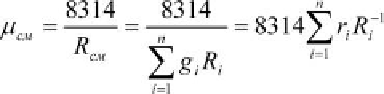




Search WWH ::

Custom Search