Environmental Engineering Reference
In-Depth Information
TABLE 2.1
The Molecular Weight
of Some Gases
gas
ammonia
air
Ar
N
CO
H
2
O
2
CO
2
m
28
40
2
32
28
44
17
29
Therefore, the internal pressure
is proportional to the square of the con-
centration
n
:
,
where, r-the gas density.
Thus, the total pressure consists of internal and external pressures:
a
pp
+ =+
p
v
H
2
Equation (2.3) is the most common for an ideal gas.
Under
normal
physical
conditions
, and then from Eq. (2.3) we obtain:
Knowing
R
we can find the gas constant for any gas with the help of the
value of its molecular mass
m
(Table 2.1):
8314
R
=
mm
For gas mixture with mass
M
state equation has the form:
8314
MT
pv
MR
T
CM
(2.4)
ì
CM
where -gas constant of the mixture.
The gas mixture can be given by the mass proportions
g
i
, voluminous
r
i
or
mole fractions
n
i
, respectively, which are defined as the ratio of mass
m
i
, volume
v
i
or number of moles
N
i
of
i
gas to total mass
M
, volume
v
or number of moles
N
of gas mixture. Mass fraction of component is
m
i
i
=
1
n
g
i
=
, where
.
It is
M

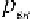


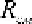

























Search WWH ::

Custom Search