Chemistry Reference
In-Depth Information
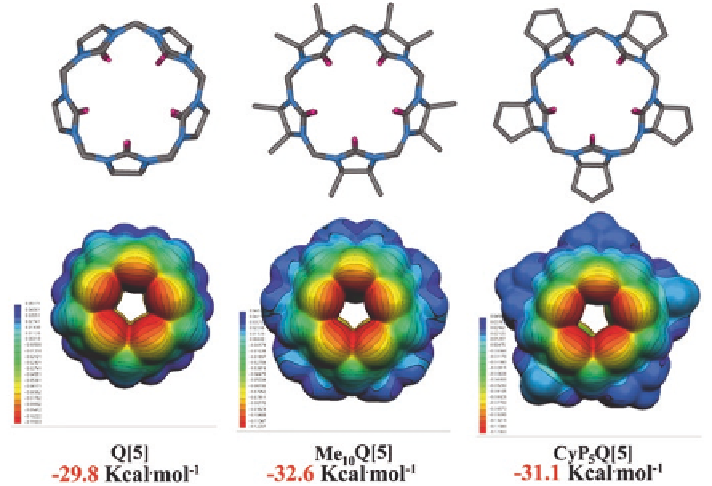
Fig. 3.7
Electrostatic potential maps (ESPs) for Q[5], Me
10
Q[5], and CyP
5
Q[5], respec-
tively. ESPs are mapped on electron density isosurfaces (0.001 e/au
3
) for cucurbit[
n
]urils at the
B3LYP/6-311G (d, p) level of theory with Gaussian09
rings (Scheme
3.1
a), which may further fuse into 2D networks (Scheme
3.1
b).
The first SQ[5]-based supramolecular ring was constructed from
ʱ
,
ʱ
′-
dimethylcucurbit[5]uril (DMeQ[5] though direct coordination of metal ions to the
carbonyl oxygen of the substituted glycoluril moieties of DMeQ[5] [
18
]. Further
studies have revealed that it is a common feature of the alkyl-substituted SQ[5]s to
coordinate directly with metal ions and to form a SQ[5]-based supramolecular ring
which can fuse into various SQ[5]-based metal-organic networks (Fig.
3.8
) [
18
].
In all of these cases, a common structural motif, i.e., a trigonal-planar branch, can
be observed. Each branch contains 12 metal ions (the metal ion is K
+
) coordinated
in a similar way and three SQ[5] moieties; each K
+
ion not only coordinates to
the portal carbonyl oxygens of an SQ[5], but also directly coordinates to one of
the carbonyl oxygens of the neighboring SQ[5]. The branches can fuse into six-
membered “bracelets,” and these can further fuse into 2D networks (Scheme
3.1
b
and Fig.
3.8
).
X-ray crystal structures show that these SQ[5]/K
+
-based networks are assem-
bled from trigonal-planar branches in which each of three SQ[5] “beads” is linked
by K
+
ions, as shown in Fig.
3.8
. Six-membered rings with different geometric
patterns can be identified in the corresponding 2D SQ[5]/K
+
-based networks.
These patterns include trigonal (Fig.
3.8
d, q), hexagonal (Fig.
3.8
c), and quadrilat-
eral patterns (Fig.
3.8
h, u). At first glance, all five trigonal-planar branches of the
respective compounds are structurally similar. In reality, however, they have many
Search WWH ::

Custom Search