Chemistry Reference
In-Depth Information
3.2 Induced Coordination Polymers of Cucurbit[5]urils
with Metal Ions
As mentioned already, there has been a trend toward the use of third species as
structure directing agents in Q[
n
]-metal systems, and the driving forces could be
the so-called outer surface interactions of Q[
n
]s [
13
]. These species produce Q[
n
]-
based supramolecular assemblies whose properties, structural novelties, and func-
tionalities exceed those of assemblies obtained in the absence of such agents. For
example, the coordination behavior of the light lanthanides can sometimes show
a marked difference to that of the heavier lanthanides. This was clearly demon-
strated in a study by Thuéry [
14
] who attempted to prepare a similar K
+
/Ln
3
+
complex of the “heavy” lanthanide ion, Yb
3
+
, by employing a corresponding
Yb
3
+
/K
+
-Q[5] perrhenic acid reaction mixture that is used to prepare the hetero-
metallic Ln
3
+
/K
+
-Q[5] complexes of Ce
3
+
, Sm
3
+
, Gd
3
+
(represented schemati-
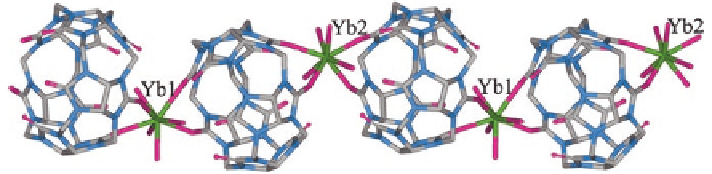
a colorless crystalline product whose X-ray structure showed it to be a 1D coor-
dination polymer of type {[Yb(Q[5])(H
2
O)
4
](ReO
4
)
3
H
2
O}
n
. Unexpectedly, K
+
does not coordinate, while Yb
3
+
ions bind simultaneously to adjacent Q[5] portals
so that Yb
3
+
-Q[5]-Yb
3
+
-Q[5]-Yb
3
+
- metallo-supramolecular chains are gen-
erated (Fig.
3.5
); once again the [ReO
4
]
−
counter ions occupy positions between
the chains. In discussing his results, Thuéry pointed out that both the presence of
K
+
and perrhenic acid in the reaction medium are important for the successful iso-
lation of both the heteronuclear Ln
3
+
/K
+
-Q[5] complexes and the homonuclear
polymeric Yb
3
+
-Q[5] complex [
14
].
Although we have not observed linear coordination polymers by simply mixing
the unsubstituted Q[5] with alkali or alkaline earth metal salts other than potas-
sium salts, we obtained such supramolecular polymers constructed from SQ[5]s
with other metal ions by simply mixing the two species. For example, the reac-
tion of CyH
5
Q[5] with NaCl can give a 1D coordination polymer (Fig.
3.6
a)
[
15
]. Close inspection reveals that Na1 and Na2 also fully cover the two por-
tals of a CyH
5
Q[5] molecule, resulting in the formation of typical molecular
capsules. These capsules are linked by the third sodium cations (Na3) through
direct coordination with two neighboring CyH
5
Q[5] molecules, and form the 1D
Fig. 3.5
X-ray crystal structure of the Yb
3
+
-Q[5] 1D coordination chain polymer present in
{[Yb(Q[5])(H
2
O)
4
](ReO
4
)
3
H
2
O}
n
Search WWH ::

Custom Search