Chemistry Reference
In-Depth Information
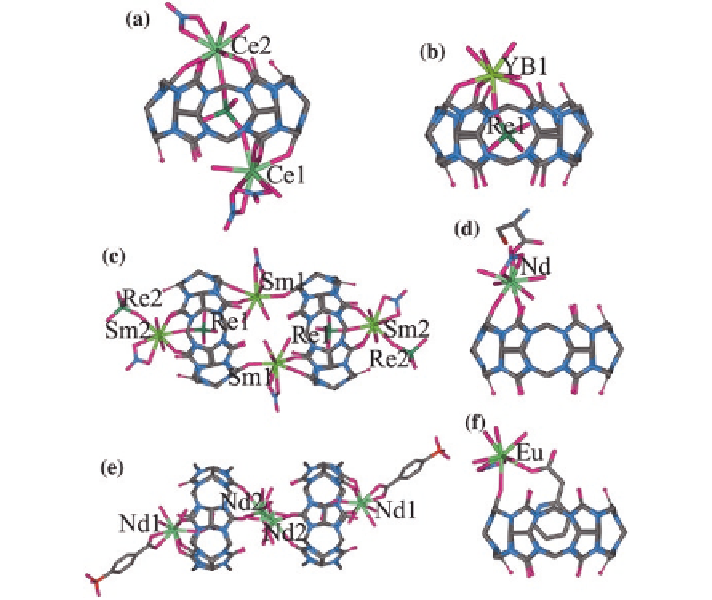
second metal salts. For example, Thuéry characterized a series of Q[6]/Ln
3
+
-based
molecular capsules (Ln
=
Ce
3
+
, Pr
3
+
) and molecular bowls (Ln
=
Yb
3
+
, Lu
3
+
)
including a perrhenate anion in perrhenic acid (Fig.
2.21
a, b). Only the coordina-
tion of Sm
3
+
cations with Q[6] form a molecular pair in which two Sm
3
+
cati-
ons link two neighboring Q[6] molecules (Fig.
2.21
c) [
53
]. In the presence of
the
ʱ
-amino acid L-cysteine (L-cys), the reaction of Nd(NO
3
)
3
, Eu(NO
3
)
3
, or
Tb(NO
3
)
3
salts with Q[6] gives simple complexes that belong to Q[
n
]-based
each metal cation is bound to the bidentate Q[6] and the monodentate L-cys mol-
ecules, with the latter in the zwitterionic form, and the ammonium group of L-cys
is directed away from Q[6] (Fig.
2.21
d) [
27
]. In the presence of
p
-sulfobenzoic
acid (4-SB), combination of Q[6] with Nd(NO
3
)
3
seems to give a molecular pair
in which two Q[6]-based capsules are close to each other (the two Nd
3
+
cations
between the two capsules have 50 % occupancy). However, the Nd
3
+
cation at the
other portal of Q[6] coordinates to both the Q[6] molecule and 4-SB (Fig.
2.21
e)
[
53
]. It is interesting to compare the former two Q[6]/Ln
3
+
/guest complexes
Fig. 2.21
X-ray crystal structures of simple complexes of Q[6]/Ln
3
+
cations and the correspond-
ing supramolecular assemblies:
a
a molecular capsule of Q[6]/Ln
3
+
(Ln
=
Ce, Pr);
b
a molecular
bowl of Q[6]/Ln
3
+
(Ln
=
Yb, Lu),
c
a molecular pair of Q[6]/Sm
3
+
;
d
a Q[6]/Ln
3
+
/L-cys com-
plex (Ln
=
Nd, Eu, Tb);
e
a Q[6]/Nd
3
+
4-SB complex pair; and
f
a Q[6]/Eu
3
+
/HPA complex
Search WWH ::

Custom Search