Chemistry Reference
In-Depth Information
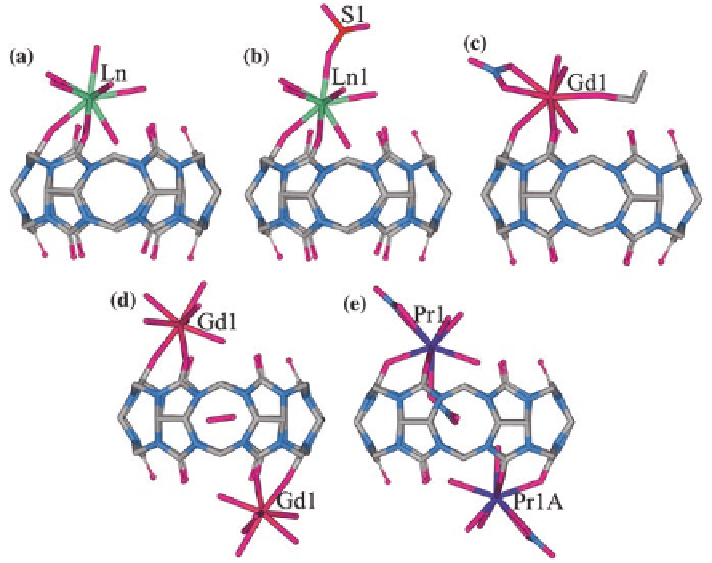
these products were isolated either directly from water or following diffusion of
ethanol into their aqueous solution. While the X-ray structure of (i) was not deter-
mined, the 1:1 (Ln
3
+
:Q[6]) complexes (ii) and (iii) were shown to be isostructural
(Fig.
2.17
a). In the case of (iv), the reaction solution contained lanthanum nitrate,
Q[6], and copper sulfate. The X-ray structure of the latter product showed that the
complex consists of an La
3
+
bound to a portal of Q[6] via two oxygens, with the
nine-coordinate coordination sphere of the metal center being completed by six
water molecules and a monodentate sulfate ligand (Fig.
2.17
b). The X-ray struc-
ture of [Gd(NO
3
)(C
2
H
5
OH)(H
2
O)
2
(H
2
O@(Q[6])](NO
3
)
2
5.5H
2
O (v), which
crystallized from the reaction solution following the slow diffusion of alcohol,
showed that the Gd
3
+
is eight-coordinate with the coordination sphere consist-
ing of a bidentate Q[6] unit, an ethanol molecule, a bidentate nitrate, and three
aqua ligands (Fig.
2.17
c) [
45
]. This product incorporates one water molecule in
the Q[6] cavity (not shown in Fig.
2.17
c). In a related study, it was shown that
Gd
3
+
chloride reacts with Q[6] to yield {[Gd(H
2
O)
6
]
2
[(H
2
O@Q[6])}Cl
6
4H
2
O
in which Q[6] acts as a bis-bidentate ligand such that a pair of carbonyl oxygens
Fig. 2.17
X-ray crystal structures of selected Ln
3
+
-Q[6] complexes prepared from La
3
+
nitrate
salts:
a
the isostructural complexes [Ho(NO
3
)(H
2
O)
4
(Q[6])]
2
+
and [Yb(NO
3
)(H
2
O)
4
](Q[6])]
2
+
;
+
b
[La(H
2
O)
6
(SO
4
)(Q[6])]
with bound sulfate group;
c
[Gd(NO
3
)(C
2
H
5
OH)(H
2
O)
2
(H
2
O@
Q[6])]
2
+
with coordinated ethanol molecule, the included water molecule is not shown;
d
{[Gd
(H
2
O)
6
]
2
[(H
2
O@Q[6])}
6
+
, included water molecule not shown;
e
[{Pr(NO
3
)
2
(H
2
O)
3
} {Pr(NO
3
)
(H
2
O)
3
(H
2
O@Q[6])}]
3
+
, the included (bound) nitrate ion and water molecule not shown
Search WWH ::

Custom Search