Chemistry Reference
In-Depth Information
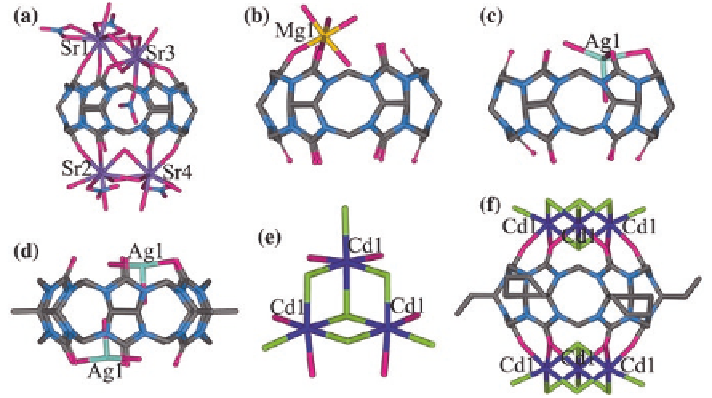
Fig. 2.14
X-ray crystal structures of
a
a Q[6]/Sr
2
+
complex;
b
a Q[6]/Mg
2
+
complex;
+
+
−
c
a Q[6]/Ag
complex;
d
a TMeQ[6]/Ag
complex;
e
a [Cd
3
Cl
3
(
ʼ
2
-Cl)
3
(
ʼ
3
-Cl)]
cluster; and
f
a CyH
6
Q[6]/Cd
2
+
complex
In aqueous solution, the interaction of Q[6] with transition metal salts prefer
to form adducts of Q[6] with metal aqua complexes through hydrogen bonding.
There are a few exceptions to the usual products, such as Q[6]/Ag
+
complexes
from a solution containing a Q[6]/isonicotinic acid/La
3
+
host-guest complex
in the presence of AgNO
3
, as shown in Fig.
2.14
c [
39
]. Another is the complex
formed by tetramethyl-substituted Q[6] (TMeQ[6]) and AgNO
3
[
40
,
41
]. In the
resulting Q[6]/Ag
+
complex, only one Ag
+
cation coordinates to two carbonyl
oxygens of a Q[6] portal, whereas in the TMeQ[6]/Ag
+
complex, both portals
of TMeQ[6] are coordinated to an Ag
+
cation and form a molecular capsule fea-
ture (Fig.
2.14
d). Another example is the complex of cyclohexanocucurbit[6]uril
(CyH
6
Q[6]) with Cd
2
+
cations [
42
]. Interestingly, two [Cd
3
Cl
3
(
ʼ
2
-Cl)
3
(
ʼ
3
-Cl)]
−
cluster anions effectively cover each portal of CyH
6
Q[6], forming one sealed
molecular capsule (Fig.
2.14
e, f).
Professor Zhang in our laboratory investigated coordination assemblies
by reactions between alkali metal salts and a
meta
-hexanomethyl-substituted
cucurbit[6]uril (
m
-HMeQ[6]) in aqueous solutions. X-ray diffraction analysis
revealed that each
m
-HMeQ[6] coordinates with three alkali metal ions, K
+
, Rb
+
,
or Cs
+
and forms a 1:2,
m
-HMeQ[6]:M
alkali
+
complex (Fig.
2.15
) [
43
].
In 2002 Fedin and coworkers reported the first lanthanide complex of Q[6]
(a Sm
3
+
species) [
44
]. The product formed on slow evaporation of an aqueous
solution of SmBr
3
and Q[6] and had an Sm
3
+
:Q[6] stoichiometry of 2:3. The
X-ray structure shows a triple-decker sandwich arrangement (height: ~33 Å) of
type {(Q[6])[Sm(H
2
O)
4
](Q[6])[Sm(H
2
O)
4
](Q[6])}
6
+
(Fig.
2.16
). Each Sm
3
+
is
coordinated by four waters and two Q[6] units. Relative to Q[5], Q[6] has a larger
Search WWH ::

Custom Search