Chemistry Reference
In-Depth Information
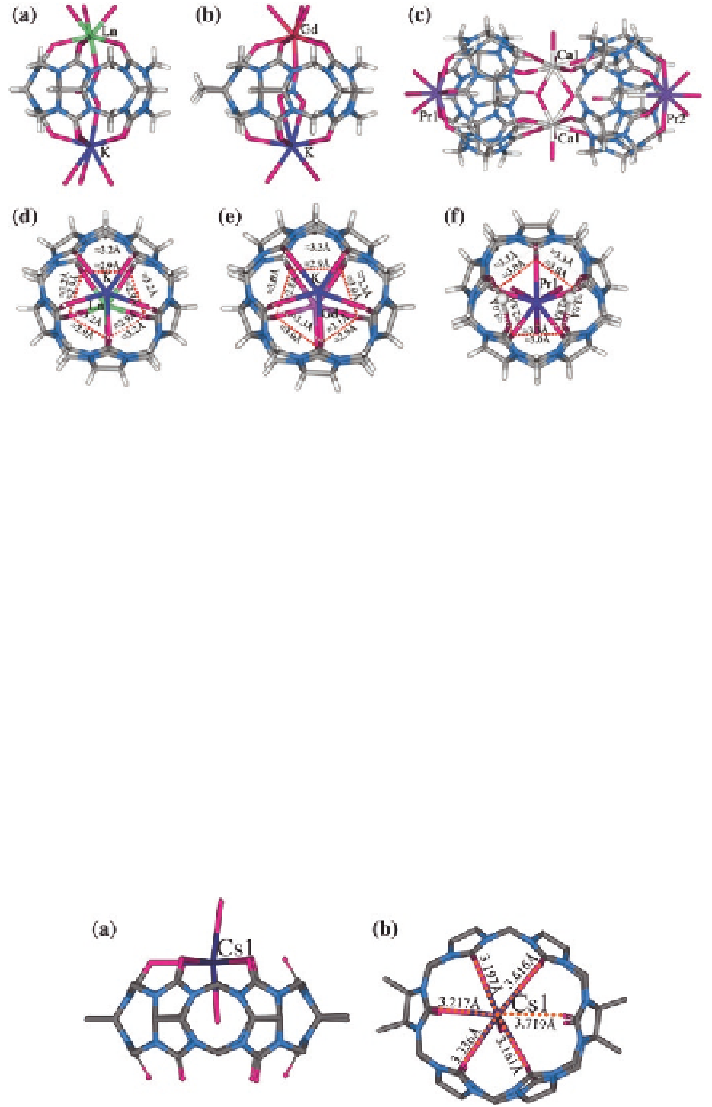
Fig. 2.11
X-ray crystal structures of heterometallic Ln
3
+
+
Q[5] complexes:
a
Ln
3
+
+
and K
/K
-
Q[5] (Ln
=
Ce, Nd, Sm, Gd);
b
Gd
3
+
+
-DMeQ[5];
c
(Pr
2
+
)
2
/(Ca
2
+
/K
)
2
-2Q[5];
d
-
f
comparison
of the portal sizes in the complexes
a
-
c
, respectively
2.2 Simple Coordination Complexes of Cucurbit[6]urils
with Metal Ions
Compared with Q[5]s, Q[6]s have a larger portal size. The portal (
r
) radius of
Q[6] molecules is within 3.3-3.4 Å, similar to the longest M
Cs
-O
carbonyl
bond
lengths (3.0-3.3 Å) observed in CyH
5
Q[5]/Cs
+
or Me
10
Q[5]/Cs
+
complexes.
Thus, it is believed that no other single metal ion can fully cover the portal of the
Q[6] molecule except the Cs
+
cation, which has the second longest ionic radius.
For example, we reported a simple complex of methyl-substituted Q[6], namely
symmetrical
ʱ
,
ʱ
′,
ʴ
,
ʴ
′-tetramethylcucurbit[6]uril (TMeQ[6]) with a Cs
+
cation
(Fig.
2.12
) [
31
]. The Cs1 atom seems to cover a portal of the TMeQ[6] molecule,
and coordinates to five portal carbonyl oxygens. The length of the bond between
+
Fig. 2.12
X-ray crystal structures of the TMeQ[6]/Cs
complex:
a
side view;
b
top view
Search WWH ::

Custom Search