Chemistry Reference
In-Depth Information
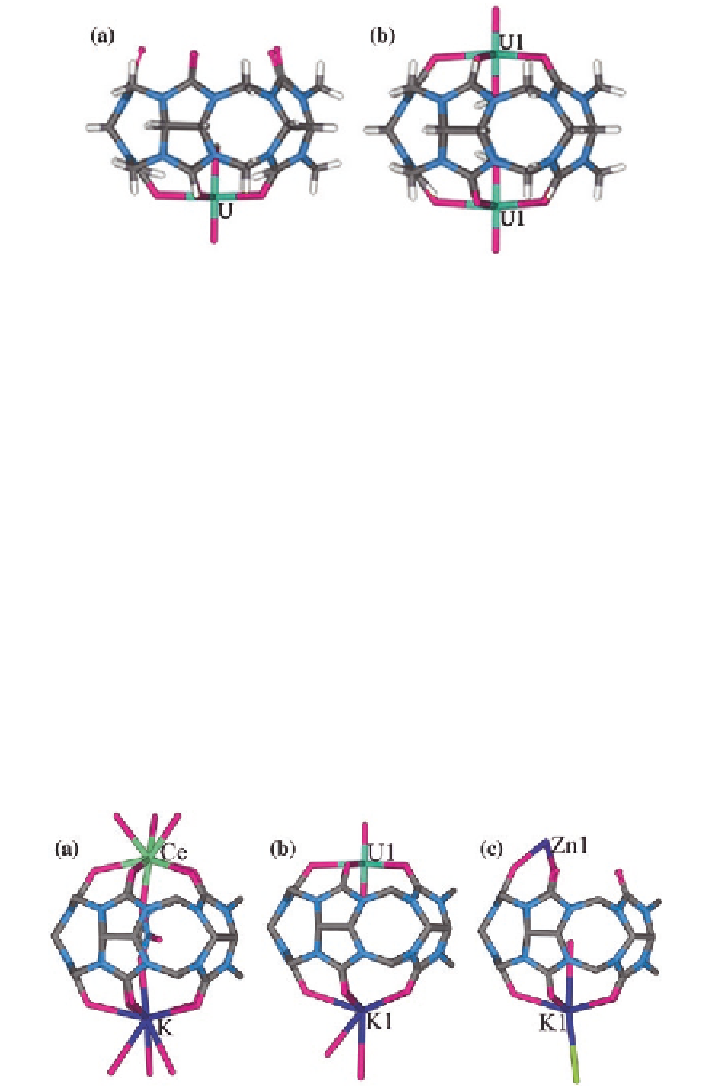
Fig. 2.9
X-ray crystal structures of Q[5] with uranyl ions:
a
molecular bowl;
b
molecular capsule
by mixing Q[5] with excess UO
3
in an aqueous HReO
4
solution. The other, with
a stoichiometry of {(UO
2
)
2
Q[5]}(NO
3
)
4
4HNO
3
3H
2
O, was prepared by mix-
ing Q[5] with excess of (UO
2
)
2
NO
3
in an aqueous HNO
3
solution. The Q[5]/UO
2
complexes had characteristic features of the molecular bowl [
27
] and molecular
capsule [
28
] respectively (Fig.
2.9
).
On the other hand, addition of the second metal ion as a structure directing
agent could also result in the formation of unusual complexes or novel Q[
n
]-based
supramolecular assemblies. For example, when Thuéry investigated the inclu-
sion properties of Q[
n
]/Ln
3
+
complexes for the perrhenate anion, he introduced
potassium cation (KNO
3
) into the Q[
n
]-Ln
3
+
-ReO
4
system. A series of Ln
3
+
/
K
+
heterometallic capped Q[5]-based capsules were formed (Ln
=
Ce, Sm, Gd),
but no suitable crystal could be obtained in the absence of the KNO
3
reactant in
this series (Fig.
2.10
a) [
27
]. In his previous work, U
6
+
/K
+
or Cs
+
heterometal-
lic capped Q[5]-based capsules were obtained by introducing alkali metal ions in
the form of their salts, such as KNO
3
or CsNO
3
, into a Q[5]-UO
2
(NO
3
)
2
6H
2
O
system (Fig.
2.10
b) [
28
]. Our group also discovered some heterometallic capped
Q[5]-based capsules, for example, a Zn
2
+
/K
+
Q[5]-based capsule that could be
−
+
/Ce
3
+
Fig. 2.10
X-ray crystal structures of heterometallic capped molecular capsules:
a
K
complex and
b
K
+
/U
6
+
+
/Zn
2
+
complex; and molecular bowl
c
K
complex
Search WWH ::

Custom Search