Chemistry Reference
In-Depth Information
four complexes include a water molecule instead of an anion in the cavity of the
Me
10
Q[5] molecule. In other words, the opened or closed molecular capsules in
the four compounds could not recognize or encapsulate the chloride anion. A simi-
lar structural feature can be observed in the complexes of SQ[5]s with alkaline
earth metal ions [
25
].
Although the first reported Ln
3
+
-Q[5] complex of this type was {(LaCl@Q[5])
LaCl(H
2
O)
9
}Cl
4
HCl 7H
2
O, obtained from an HCl solution (~3 M) containing
La(NO
3
)
2
and Q[5], La
3
+
cation has 14 lanthanide group members, which could
show similar interaction properties with Q[5]s due to the so-called lanthanide
contraction. The La
3
+
cation fully caps one portal of the Q[5] molecule, while a
second La
3
+
partially caps the second portal via coordination to two carbonyl oxy-
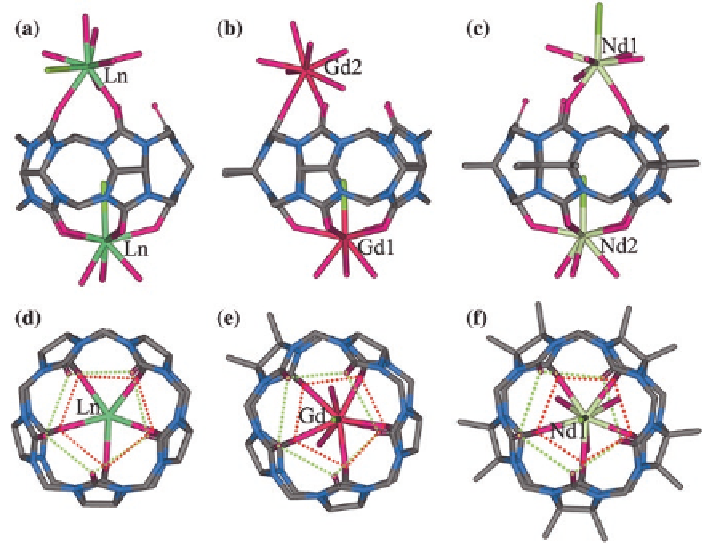
gens and a chloride ion is included in the Q[5] cavity (Fig.
2.3
d) [
18
]. A similar
arrangement occurs in the product obtained from the coordination of Pr
3
+
to Q[5]
[
26
]. The coordination of Gd
3
+
to the dimethyl-substituted derivative (Me
2
Q[5]),
or Nd
3
+
to the decamethyl Me
10
Q[5] derivative, once again results in products
exhibiting similar structural arrangements (Fig.
2.8
b and c respectively) [
16
].
In recent years, Thuéry focused on the coordination of Q[
n
]s with lanthanide
and actinide cations and synthesized two Q[5]/UO
2
complexes. One of these com-
plexes, which has a stoichiometry of {UO
2
Q[5]}(ReO
4
)
2
2H
2
O, was prepared
Fig. 2.8
X-ray crystal structures of the complexes of selected Ln
3
+
cations with Q[5] and its
Me
2
Q[5] and Me
10
Q[5] substituted derivatives:
a
Ln
3
+
-Q[5] (Ln
=
La, Pr);
b
Gd
3
+
-Me
2
Q[5];
c
Nd
3
+
-Me
10
Q[5];
d
-
f
the portal sizes of the corresponding complexes
Search WWH ::

Custom Search