Information Technology Reference
In-Depth Information
Neutral DBs
Negative DBs
(a)
(b)
High doped n-type Silicon
Low doped n-type Silicon
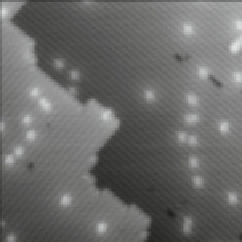
Fig. 4.
Two silicon surfaces imaged under the same conditions. (a) A moderately
n-type doped sample where DBs are on average neutral. (b) A highly doped sample
where DBs are on average negatively charged.
negative charge at a DB causes destabilization of electron energy levels referred
to as upward band bending. To a first approximation it is the inaccessibility
of empty states for the STM tip to tunnel into that causes the highly local
darkening of the STM image, that is, the halo. A much fuller description of the
competing process involved in the imaging process have been described [
15
].
It is evident that the DB is effectively a dopant with a deep acceptor level.
In accord with that character, a DB acts to compensate bulk n-type doping,
causing the bands to shift up with respect to the Fermi level in the direction
of a p-doped material. Most recently it has been shown that the neutral, single
electron occupied DB can donate its charge to become positive thereby acting
as a deep n-type dopant [
16
]. Summarizing, single electron occupation corre-
sponds to neutral state. Two electron occupation corresponds to 1
charge. The
absence of electrons in the DB leaves it in a 1+ charge state. The combination
of dopant type, concentration, DB concentration on the surface, local electric
field, and finally current directed through a DB, all contribute to determining
its instantaneous charge state [
15
].
A clean silicon surface, where every site has a dangling bond, is very reactive
toward water, oxygen and unsaturated hydrocarbons like ethylene and benzene.
While H atoms immediately react with a clean silicon surface, H
2
does not
[
17
]. It is a remarkable fact that single DBs interact only weakly with most
molecules, resulting in no attachment at room temperature. Most often, a second
immediately adjacent DB is required in order for a molecule to become firmly
bonded to the surface. Two DBs typically act together to form two strong bonds
to an incoming molecule. This has the practical consequence that a protective
layer can be formulated and applied to encapsulate and stabilizes DBs against
environmental degradation.
A special class of molecules, typified by styrene, C
8
H
8
attach to silicon via a
self-directed, chain reaction growth mechanism [
18
]. As shown in Fig.
5
a, a ter-
minal C reacts with a DB, thereby creating an unpaired electron at the adjacent
C on the molecule. That species follows one of two paths. It either desorbs, or the
−




Search WWH ::

Custom Search