Environmental Engineering Reference
In-Depth Information
Q
+
−
Q
+
+
+
Q
M
1
M
2
hv
S*
S
M
3
P
A
B
B
P
A
P
Fig. 8
Nature of photoreactions in micellar systems.
S
, pesticide;
P
, photoproduct; [
A• •B
], paired
radicals in a solvent cage;
A
• and
B
•, free radicals;
M
1
, reactant solubilized in micelle;
M
2
, weakly
micelle-associated reactant;
M
3
, reactant in an aqueous phase;
Q
, quencher solubilized in micelle;
Q
+
and
Q
−
, ionic quenchers.
Dashed
and
solid arrows
, diffusion and reaction processes,
respectively
(Fendler and Fendler 1975). When the energy donor and acceptor coexist in the
same micelle, the efficiency of energy transfer would increase.
Based on the Poisson distribution, most micelles are considered to be free from
molecular oxygen under the usual conditions of [O
2
] = 10
−4
M and [micelle] =
1 mM. The characteristics of a micelle result in several effects on photoreactions,
the most popular one being a cage effect (Ramamurthy 1986). The reactive species
such as radicals (A
.
and B
.
in Fig. 8) produced by photoinduced bond cleavage of
a solute molecule spends more time in the restricted micellar space than in homo-
geneous solution, which alters the profiles of radical recombination. The product
distribution in the Norrish type I and II reactions of benzoin methyl ether signifi-
cantly varied with the type of surfactant-forming micelles. The orientational effect
also controls the product distribution in various photoreactions (Turro et al. 1980).
The position of intermolecular hydrogen abstraction from the alkyl chain of sur-
factant by the excited benzophenone derivatives was found to be dependent on the
locus of the carbonyl group in micelles. The regioselectivity in photo-induced








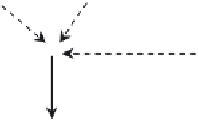


Search WWH ::

Custom Search