Environmental Engineering Reference
In-Depth Information
Table 5
Hydrolysis of functional moieties of organic chemicals in micelles
Functional moiety/structure Surfactant/medium
T (°C)
K
s
a
k
2w
b
k
2m
c
Reference
Carboxylic ester
R
2
OC
R
1
O
R
1
=CH
3
, R
2
=H
HDTMA Br (
30 mM)
+ 4.7 mM OH
−
≤
30
85
3.2
0.235
Broxton et al. (1988)
HDTMA SO
4
(<16 mM)
55
3.2
0.182
R
1
=C
11
H
23
, R
2
=H
HDTMA Br (0.1-20 mM)
at pH 11.66
25
2.5 × 10
3
*
1.8 × 10
−6
*
4.6 × 10
−4
Al-Awadi and Williams (1990)
R
1
=C
11
H
23
, R
2
=4-Me
(1-30 mM)
2.4 × 10
3
*
1.0 × 10
−6
*
4.0 × 10
−4
R
1
=C
11
H
23
, R
2
=4-Cl
(1-20 mM)
2.6 × 10
3
*
4.6 × 10
−6
*
1.3 × 10
−3
R
1
=C
11
H
23
, R
2
=4-NO
2
(1-45 mM)
2.4 × 10
3
*
8.2 × 10
−5
*
2.2 × 10
−2
Amide and anilides
CH
3
N
Ar
C
O
R
R=2-NO
2
, Ar=Ph
HDTMA Br (<20 mM)
+ 4.7 mM OH
−
70
200
1.69 × 10
−3
1.4 × 10
−3
Broxton et al. (1988)
HDTMA SO
4
(<30 mM)
90
1.69 × 10
−3
8.4 × 10
−4
R= H, Ar=Ph
HDTMA Br (<10 mM)
+ 5.8 mM OH
−
65.5
172
1.1 × 10
−4
1.3 × 10
−5
Broxton and Duddy (1979)
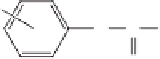
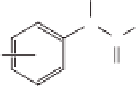


Search WWH ::

Custom Search