Environmental Engineering Reference
In-Depth Information
(b)
K
s
S + M
S · M
k
m
k
w
P
P
(a)
Region 2
Region 1
[Surfactant]
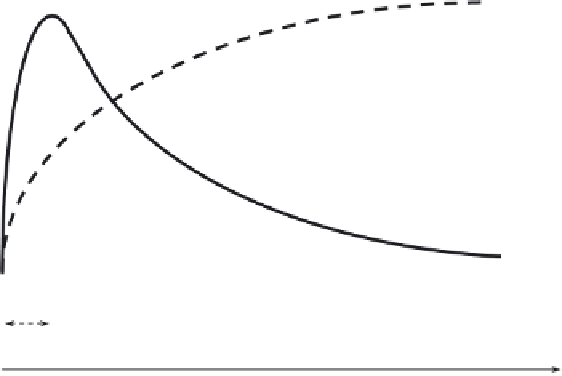
Fig. 7
Base-catalyzed hydrolysis in the presence of cationic surfactant. (a) No adjustment of a
counterion concentration. (b) Under the constant concentration of a counterion.
S
, substrate;
M
, micelle;
M.S
, micelle-substrate complex;
P
, product;
K
s
, micelle-substrate partition constant;
k
w
, rate constant in an aqueous phase; k
m
, rate constant in a micellar phase
N and X are the inert counter ion such as Br
−
and the reactive ion such as H
+
and
OH
−
, respectively. The subscripts w and m represent the bulk water and micellar
phases. The
b
-value is known to be in the range of 0.6-0.9. When the fraction of X
in micelles (m
x
= [X
m
] / ([S] - cmc)) is used, k
obs
can be newly expressed below. [N]
and [X] are the total concentrations in the system.
k
obs
= {k
2w
.
[X
w
] + k
2m
.
m
x
.
K
s
([S] − cmc)} / {1 +
K
s
([S] − cmc)}
k
w
= k
2w
.
[X
w
], k
m
= k
2m
.
m
x
m
x
2
(
K
N
X
− 1) ([S] − cmc) + m
x
{[X] +
K
N
X
[N] −
b
(
K
N
X
−1) ([S] − cmc)}−
b
[X] = 0
where k
2w
and k
2m
are the second-order reaction rates in the aqueous and micellar
phases, respectively. The apparent base-catalyzed reaction rate in cationic micelles
generally increases, as shown in Fig. 7 (solid line, region 1), and afterward
decreases (region 2), which can be described by the above equation. The k
2w
and
k
2m
values are mostly of similar order and, therefore, the observed rate enhancement
is not a real catalysis but originates from the concentration effect. It is considered



Search WWH ::

Custom Search