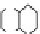Environmental Engineering Reference
In-Depth Information
HO
O
CO
CH
HO
HC
CO
O
OH
OH
O
H
OH
OH
CI
HO
O
CI
CI
CI
HO
O
CO
CH
+ 6 O
2
+ 5 H
2
O
12 CO
2
+ 12 HCL
2
CI
HO
>250
o
C
HC
CO
O
OH
HO
CH
Pentaclorfenol
(creozol)
OH
HO
HO
O
CO
CH
2
pentagailoilglucoz ˆ
HO
HO
HO
CH
CH
HO
CH
CH
OH
+ 4 O
2
O
O
HO
HO
OH
HO
HO
CO
O
OH
OH
6
+ 2
+ 10 CO
2
OH
OH
2
O
>250
o
C
O
O
HO
OH
HO
CH
digaloilendioxina galoilen m-cresilendioxina
ˆ
ˆ
CH
2
CO
O
OH
OH
+ 36 HCI
+ 10 HCI
OH
digaloilfructoza
taninat de Hamameli
ˆ
>250
o
C
>250
o
C
HO
HO
H
O
HO
O
CH
CO
CH
OH
CI
O
O
O
CI
CI
CI
CI
CI
O
CO
CH
2
6
+ 36 H
2
O
+ 10 H
2
O
CI
CI
HO
O
O
CH
CH
OH
CI
CI
CI
HO
H
O
hexaclordibenzodioxina
(HxCDD)
ˆ
pentaclordibenzidioxina
(PCDD)
ˆ
HO
HO
O
CO
CH
2
ˆ
corilagina
tanin
Fig. 4.3 Conversion of tannins to dioxins in the combustion of railroad ties made of creosote-
treated wood [
22
,
23
]
comprises a large number of halogenated solvents and pesticides of the HCH and
DDT type [
25
,
26
]. More recent research studies indicate other involuntary ways of
generating POPs-type compounds [
27
] (Fig.
4.4
).
Conversion through combustion of polychlorinated biphenyls (PCB and PCT) to
dioxins and furans is shown in Fig.
4.5
[
21
,
22
].
Combustion of highly chlorinated polymers such as polyvinyl chloride (PVC)
may also produce dioxins and furans (Fig.
4.6
)[
22
,
24
,
27
].
Dioxins and furans persist as particulate matter in the atmosphere, water or soil
not only because they are chemically stable and easy to form during any combus-
tion process, but also because they tend to reform from smoke gas, through the slow
cooling of smoke (Figs.
4.4
and
4.7
)[
21
,
22
,
28
].
Most certainly, the environment contains many other nanoparticle species of
organic nature such as organometallic compounds (methyl and ethylmercury, heavy
metal phenoxides or other cyclic derivatives with more or less complex molecules)
whose formation and persistence mechanisms are topics of current debate and
research [
29
].