Environmental Engineering Reference
In-Depth Information
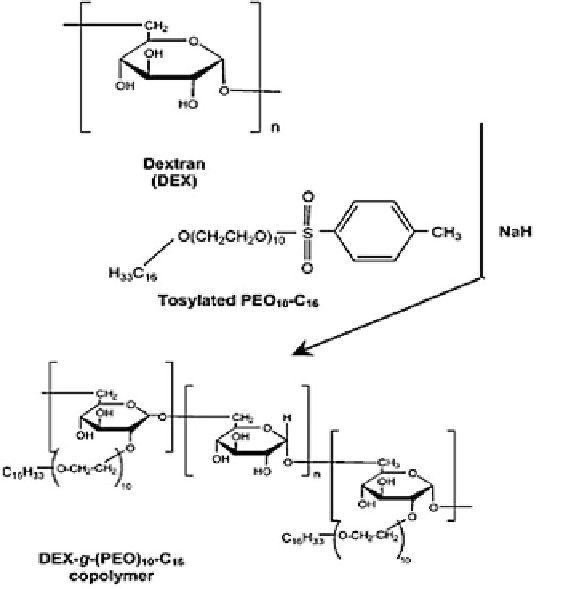
Fig. 16.2 Synthesis of DEX-
g
-PEO
10
-C
16
copolymer [
68
]
Trimethyl chitosan polymers can be synthesized by reductive methylation
of chitosan by the chemical reaction between chitosan,
N
-methyl-2
pyrrolidinone (NMP), and iodomethane in the presence of sodium hydroxide
(Figs.
16.2
and
16.3
)[
70
].
A 100 ml three-neck flask, equipped with a reflux condenser and a dropping
funnel, is loaded with decanoic acid (3.7 g) and heated at 84
C with an oil bath to
melt the acid. A mixture of thionyl chloride (SOCl
2
, 7.85 g) and dimethyl form-
amide (0.5 mmol) is added slowly at 84
C within 30 min. After 5 h, the product is
recovered by removing the excess SOCl
2
under vacuum conditions.
The synthetic route leading to amphiphilic
N
,
O
-acyl group-
N
-trimethyl chitosan
chloride from trimethyl chitosan (TMC) is as follows: TMC (1.0 g) is dissolved in
30 ml of the aqueous solution with trifluoroacetic acid (0.1 ml) [
71
,
72
] and the
acetone (80 ml) is added under intense stirring. Pyridine (3.9 g) dissolved in acetone
(40 ml) is first added dropwise into the mixture and then a solution of decanoyl
chloride (40 mmol) obtained by the above method in acetone (40 ml) is loaded later
on. The reaction is lasted for 12 h and then the mixture is poured into ice water,
subsequently the suspension is filtered and the filter liquor is extracted by diethyl
ether for five times, dialyzed by dialysis membranes (MWCO 10,000) against