Environmental Engineering Reference
In-Depth Information
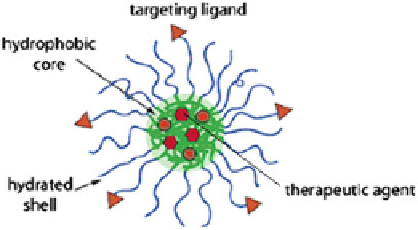
Fig. 16.1 Structure of
polymeric micelles
the carrier should be easily removed from the body when the therapeutic function is
completed. In broad terms, micelles as drug carriers provide a set of unbeatable
advantages. The solubilization of drugs using micelle-forming surfactants (which
results in formation of mixed micelles) leads to increased water solubility of a
sparingly soluble drug and its improved bioavailability, reduction of toxicity and
other adverse effects, enhanced permeability across the physiological barriers, and
substantial changes in drug biodistribution [
26
]. Micelles may be made targeted by
chemical attachment of targeting moiety to their surface. In the latter case, local
release of the loaded drug from the micelles in the target organ should lead to
increased efficacy of the drug. On the other hand, the drug is well protected in
micellar form from possible inactivation under the effect of biological surround-
ings, and does not provoke undesirable side effects on nontarget organs and tissues.
They can also be produced in a reproducible manner with a narrow particle size
distribution (Fig.
16.1
).
Owing to outer hydrophilic shell, the micelles remain stable in aqueous medium
without the need of additional stabilizer. Further, the outer hydrophilic segment
prevents easy recognition of the micelles by reticuloendothelial system (RES) and
thus prolongs their circulation time in blood and allows drugs to be administered
over prolonged periods of time. This is termed as stealth or long-circulating
micelles. For nanoparticles, it is necessary to coat the particles with additional
hydrophilic polymers (polyethylene glycol) or add nonionic surfactants (Tween
80, poloxamers) to impart it long circulating character. Hence, the design of
micellar formulation is a best alternative. The size, charge, and surface character-
istics can also be modified by variation in preparation technique.
However, one major disadvantage is that surfactant micelles rapidly break apart
upon dilution, result in premature leakage of the drug, and ultimately cause its
precipitation in situ. This limitation of surfactant micelles as drug delivery carriers
triggered the search for micelles of significantly enhanced stability and solubilizing
power. The design of biopolymer-based micelles has gained much attention
because of their biocompatibility, biodegradability, and the multiplicity of func-
tional groups they display for the conjugation of pilot molecules [
27
]. Like their
low-molecular-weight counterparts, amphiphilic polymers associate in water to
form polymeric micelles [
28
].