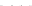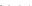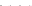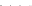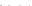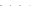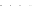Environmental Engineering Reference
In-Depth Information
[M+Na
+
]
+
55+
GLA -S
Intens.
Intens.
Intens.
GLA -S
5
841.1
x10
[M+H
+
]
+
Intens .
Intens .
684.1
58+
GLA -P
6
6
797.6
x10
x10
484.0
[M+H
+
]
+
6
GLA -S
1.25
1.25
52+
646.1
889.6
668.1
MW
exp
= 46205.5 Da
4
1.00
1.00
61+
61+
758.4
758.4
49+
944.1
[M+H
+
]
+
0.75
0.75
2
64+
64+
GLA -IS
934.6
489.2
722.9
722.9
1009.1
0.50
0.50
0
700
750
800
850
900
950
1000
545.9
Acid
ʱ
- galactosidase
713.9
0.25
0.25
944.1
1009.1
610.9
841.1
899.6
0.00
0.00
500
600
700
800
900
1000
1100
1200
1300
m/z
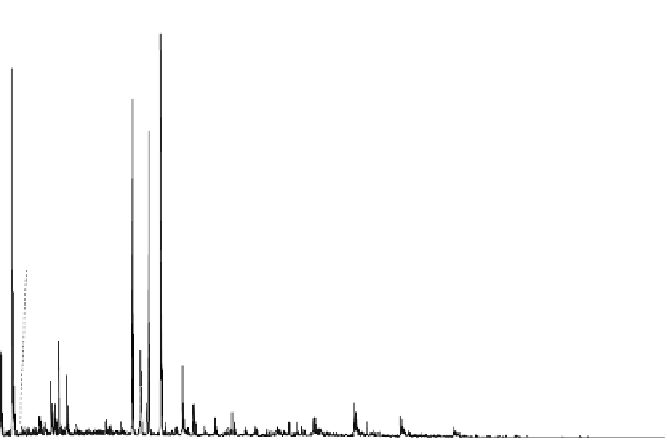
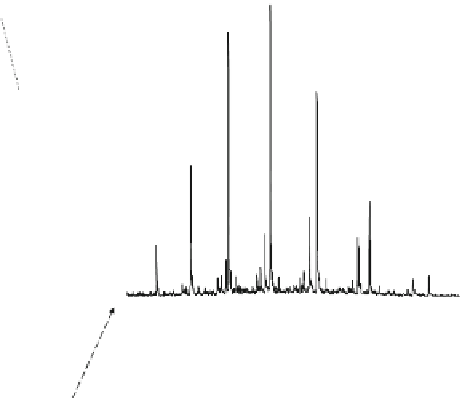
Fig. 8.7 Fully automated chip-nanoESI on a NanoMate robot coupled to HCT MS of the assay
products after enzymatic reaction in a DBS sample from a healthy donor.
Inset
: zoom out of
m/z
(720-1,010) range corresponding to
46,205.5 Da). Positive ion
mode detection; ESI MS parameters: ChipESI, 1.4 kV; Cap Exit, 50 V. Back nitrogen pressure
0.30 psi. Nitrogen nebulizer on MS at 50 psi. Reprinted with permission from [
63
]
α
-galactosidase protein (MW
exp
¼
DBS using an aqueous buffer solution; (3) enzymatic reaction between the DBS
α
-galactosidase extracts and the substrate/internal standard (GLA-S/GLA-IS) cock-
tail — molar ratio 500:1 — resulting in cleavage off the terminal galactose from
GLA-S generating the product (GLA-P). GLA-IS, the deuterated counterpart of
GLA-P, served for positive control; (4) purification steps of the final enzymatic
mixture to remove buffers, salts and detergents using silica gel; (5) fully automated
chip-nanoESI MS and tandem MS analysis of the reaction products on a high
capacity ion trap (HCT) ultra PTM mass spectrometer coupled via an
in-laboratory mounting system to a NanoMate robot incorporating ESI 400 chip
technology.
The first MS approach of
the method demanded MS screening of
the
α
-galactosidase reaction products, both in healthy control (Fig.
8.7
) and in patient
(Fig.
8.8
). The presence of GLA-P, GLA-IS and GLA-S ions is illustrated in the
spectrum from Fig.
8.7
, which results in a GLA-P/GLA-IS ratio of 26. The presence
of GLA-P in the healthy subjects discloses an active enzyme capable to cleave
GLA-S, a fact confirmed also by the presence in the spectrum of a series of signals
having an envelope shape, which is characteristic for a protein. Using the same
condition for ionization and detection, the spectrum of DBS reaction products from
Fabry patient presented a large discrepancy compared with the healthy one.