Information Technology Reference
In-Depth Information
on a sound quantum mechanical platform [11]. The Hohenberg and Kohn
[10], the founders of DFT pointed out that the total energy
E
for
N
electron
system, is a functional of electronic density,
E
(
r
).Given the electron den-
sity function ρ(
r
) in a chemical system (atom or molecule) and the energy
functional
E
(ρ), the chemical potential, μ
of that system in equilibrium has
been defi ned as the derivative of the energy with respect to the number of
electrons at fi xed molecular geometry.
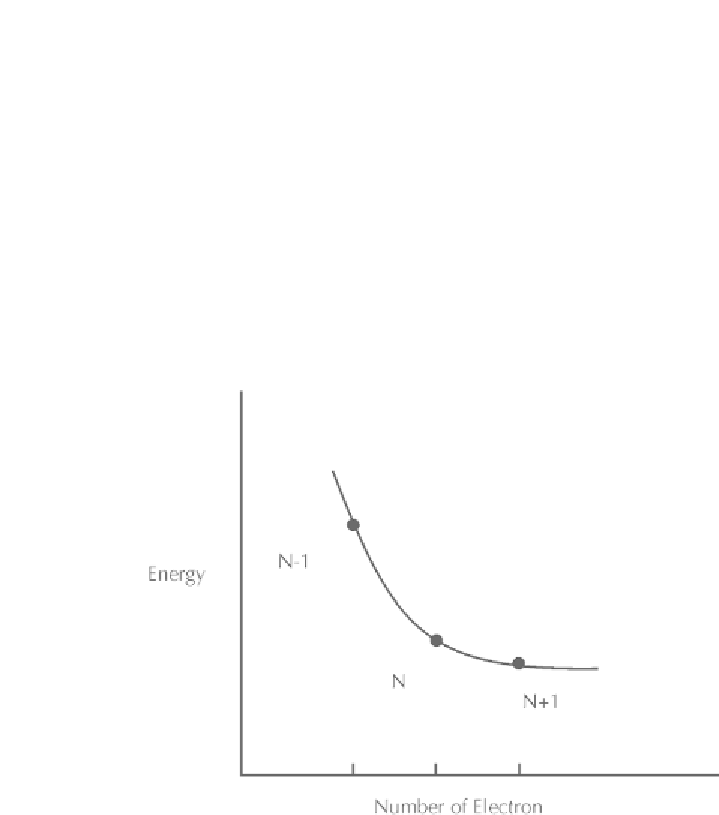
Parr et al. [43] showed that the slope, [
∂E
(
r
)/
∂N
]
v
, of the energy
E
(
r
)
versus the number of electrons (
N
) curve (Figure 1.3) at a constant ex-
ternal potential(
v
), is the chemical potential, μ, and this property such as
thermodynamic chemical potential [44] measures the escaping tendency
of electrons in the species.
FIGURE 1.3
Plot of total electronic energy (all are negative) of a system in positive (+1),
neutral (0), and negative (−1) state as a function of number of electrons (N).
The chemical potential, μ is given by [45]
μ = [δE(ρ)⁄δρ]
v
(18)
The differential defi nition more appropriate to atomic system is














Search WWH ::

Custom Search