Information Technology Reference
In-Depth Information
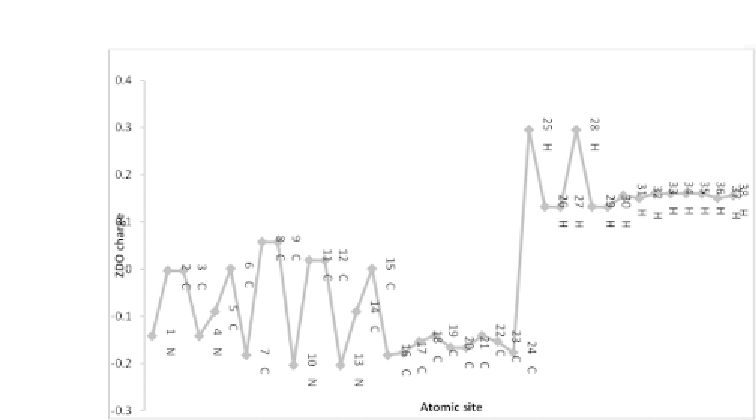
FIGURE 9.7(C)
Computed ZDO atomic charge of porphycene on the basis of AM1
calculation.
An observation on Table 9.1 reveals that the SCF and the geometrical
energy of porphycene are less than those of pyrrol. Hence, porphycene is
more stable. The heat of formation data also supports the observation.
The computed total energy (
E
), ε
HOMO
, ε
LUMO
, ionization energy (
I
),
electron affi nity (
A
), and delocalization energy (
D
) on the basis of HMO
method are presented in Table 9.4(A).
From Table 9.4(A), it is distinct that the stability of porphycene is
greater than pyrrol. The ionization energy and dipole moment of the com-
pound follows the trendporphycene < pyrrol.
Computed global reactivity parameters on the basis of HMO method
are presented in Table 9.4(B).From this table, it is transparent that the elec-
tronegativity order is pyrrol< porphycene. The hardness order is porphy-
cene < pyrrol. Of course, the softness value follows the reverse trend of the
hardness. The hardness and softness data reveal that the order of reactivity
of porphycene > pyrrol.
Electrophilicity is a property of atoms that signifi es the energy-low-
ering process on soaking the electrons from donors. The electrophilicity
index measures the stabilization in energy when the system acquires an
additional electronic charge from the environment. The elctrophilicity in-














Search WWH ::

Custom Search