Information Technology Reference
In-Depth Information
and G-BETA [D1] and C-LIKE [D2]. Only the extracellular region that corre-
sponds to these domains has been crystallized (Fig. 1). The TR V-DOMAINs and
MHC G-DOMAINs that are directly involved in TR/pMHC interactions are de-
scribed in the next sections.
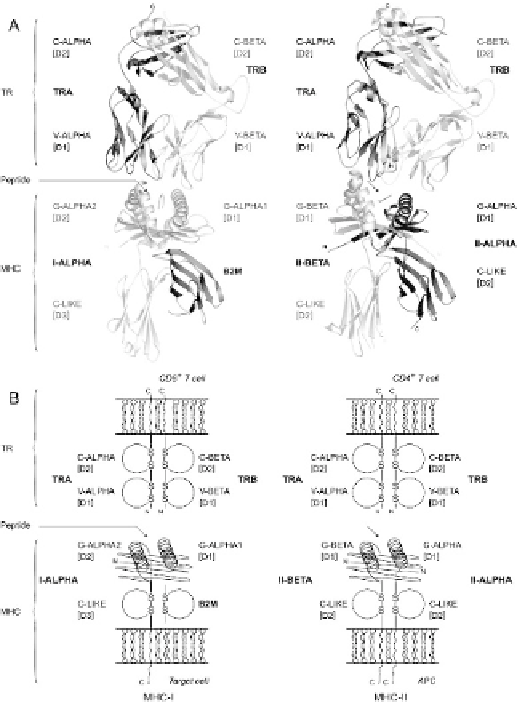
Fig. 1.
T cell receptor/peptide/MHC complexes with MHC class I (TR/pMHC-I) and MHC
class II (TR/pMHC-II). [D1], [D2] and [D3] indicate the domains. (A) 3D structures of
TR/pMHC-I (1oga) and TR/pMHC-II (1j8h). (B) Schematic representation of TR/pMHC-I
and TR/pMHC-II. The TR (TR-ALPHA and TR-BETA) chains, the MHC-I (I-ALPHA β-
2-microglobulin B2M) chains and the MHC-II (II-ALPHA and II-BETA) chains are shown
with the extracellular domains (V-ALPHA and C-ALPHA for the TR-ALPHA chain; V-
BETA and C-BETA for the TR-BETA chain; G-ALPHA1, G-ALPHA2 and C-LIKE for the I-
ALPHA chain; C-LIKE for B2M; G-ALPHA and C-LIKE for the II-ALPHA chain; II-BETA
and C-LIKE for the II-BETA chain), and the connecting, transmembrane and cytoplasmic
regions. Arrows indicate the peptide localization in the G-DOMAIN groove. The MHC G-
DOMAINs and TR V-DOMAINs are likely to be in a diagonal rather than in a verti
cal
posi-
tion relative to the cell surface (Wang, Meijers, Xiong, Liu, Sakihama, Zhang,
Joachimiak
and Reinherz 2001; Wang and Reinherz 2002).
and