Environmental Engineering Reference
In-Depth Information
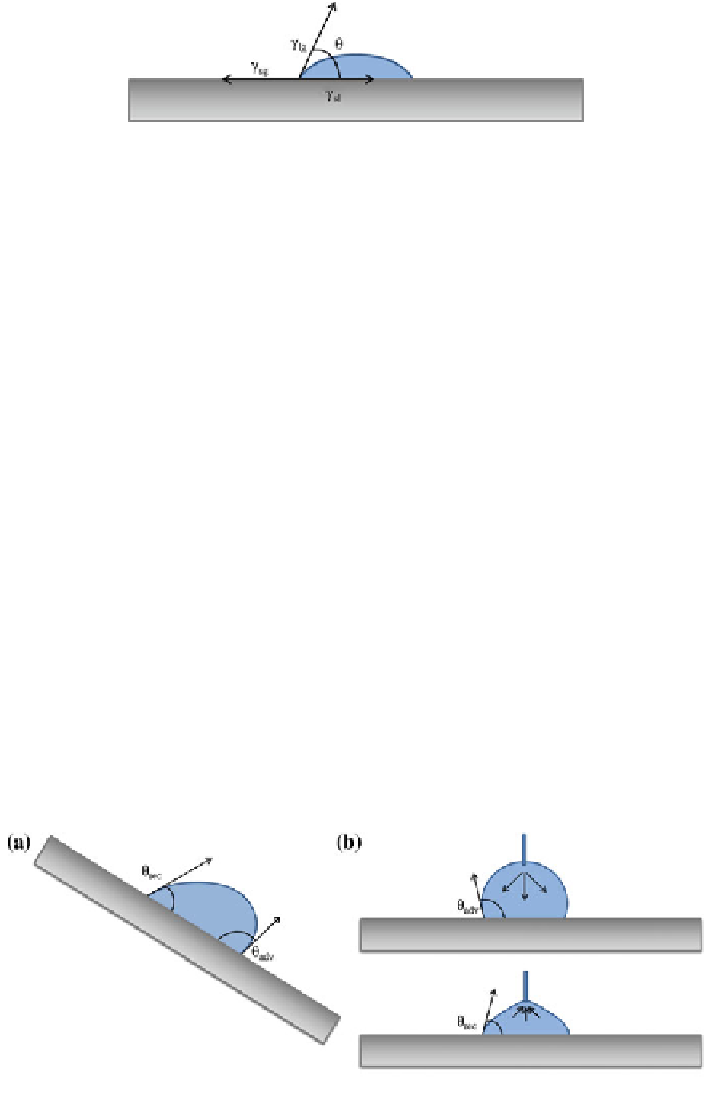
Fig. 9.3 The contact angle h formed by a liquid drop on a surface is a consequence of equilibria
among solid-gas (c
sg
), liquid-gas (c
lg
) and solid-liquid (c
sl
) surface tension
In both cases, the static contact angle h
0
that a liquid forms on a surface can be
derived by applying Young's equation (Eq.
9.1
):
cos h
0
¼
c
sg
c
sl
c
lg
ð
9
:
1
Þ
and is valid for any triple liquid-solid-gas interphase, with the gas phase generally
being air. Yet, from the point of view of self-cleaning, another fundamental aspect
of surface wettability must be considered, that is, hysteresis.
In fact, the static contact angle can only be observed on ideally flat surfaces,
while most commonly a range of values will be observed, from a minimum value
defined as receding contact angle h
rec
, to a maximum advancing contact angle h
adv
:
the difference between the two represents contact angle hysteresis. The smaller this
parameter, the easier the movement of a drop on the surface; this concept is
particularly important in the case of superhydrophobicity-driven self-cleaning,
where the cleaning effect is assured by water rolling on the surface, collecting dust
deposited on it and carrying it away (see Sect.
9.2.2
). DCA (dynamic contact
angle) is generally used to quantify it and different methods can be applied.
Although the correct observation method of hysteresis would be the one sche-
matized in Fig.
9.4
a—i.e., a drop deposited on a tilted surface, generally, first a
drop is dispensed to read the advancing angle, while the receding one is measured
by drop retraction (Fig.
9.4
b). Additionally, the material can be immersed directly
Fig. 9.4
Methods for determining advancing and receding contact angles: a tilted plane and
b dynamic contact angle
Search WWH ::

Custom Search