Environmental Engineering Reference
In-Depth Information
world, owing to the important savings that self-cleaning could bring—and has
actually brought—in the maintenance of buildings, especially in the presence of
wide glass façades: the use of self-cleaning glass and other cladding materials
surely has an impact on the overall building life cycle cost. One example is
provided on Phys.Org as a result of a market analysis performed by the Singapore
Institute of Manufacturing Technology (SIMTech), a research institute under
A*STAR (Agency for Science, Technology and Research) (Phys.Org
2008
). In
this analysis, the typical cleaning cycles of commercial buildings in Singapore are
estimated as frequent as 1-4 per year, with unit costs for energy and detergents
ranging from 7 to 40 kUSD, which can be reduced even by 75 % in the presence of
self-cleaning materials. The possibility of making surfaces more resistant to
soiling has important spin-offs not only in the built environment, but also in micro-
circuitry, where the presence of contaminants can reduce the device lifetime,
and—for the same reason—in the most recent application field of solar panels,
where even a slight reduction in transmittance can cause severe efficiency losses
(Zhu et al.
2010
).
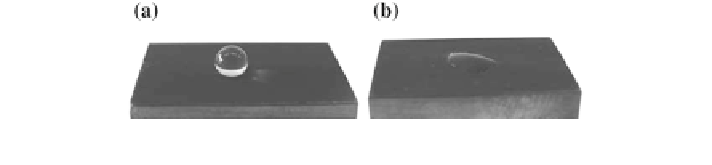
Still, the term self-cleaning may be misleading at times. In fact, it generally
refers to two situations (Fig.
9.1
):
• surfaces may show a superhydrophobic character and impede dust and soot
adhesion
and
adsorption,
due
to
peculiar
morphological
features
and/or
chemical affinity; or
• surfaces with photoactivated properties can decrease the adhesion strength of
dust and particles—as well as degrade bacterial contaminants—while
byproducts are removed by water, owing to a superhydrophilic surface state.
Moreover, some mixed mechanisms—pertaining to hydrophilic surfaces with a
relevant oleophobic, or superoleophobic, character—have been recently consid-
ered in the development of self-cleaning surfaces.
Considering first the superhydrophilicity condition, the triggering element of
such mechanism is photoactivity, which can be intrinsic of the whole material or
conferred to the sole surface by applying a suitable coating. This is the case of
titanium dioxide (TiO
2
) surfaces, whose hydrophilicity is greatly enhanced by
irradiation of suitable energy (generally in the UV range) until a superhydrophilic
state is reached; similar results can be provided by similar wide band semicon-
ductors, such as zinc oxide (ZnO) (Fujishima et al.
2008
; Khranovskyy et al.
2012
;
Zhang et al.
2012
). Although no natural structure exhibits such self-cleaning
Fig. 9.1 Example of superhydrophobic (a) and superhydrophilic (b) surfaces. Reprinted with
permission from Men et al. (
2010
)
Search WWH ::

Custom Search