Geography Reference
In-Depth Information
17.2.2.1 Egg size, fecundity
Manual measurements of oocyte number and/or sizes
imply time-consuming work. Thorsen and Kjesbu (2001)
used an IA system for estimating oocyte density, the
'auto-diametric fecundity method'. They determined the
average diameter of oocytes in a sample, and this was
converted into oocyte density using a calibration curve.
Furthermore, they showed that accurate and precise mea-
surement of oocyte size had practical implications for the
assessment of maturity stage and predictions for the start
of spawning. To estimate fecundity without sacrificing the
fish, Will et al. (2002) assessed ovary volume from ultra-
sonic imaging, as in medical imagery. The total length of
the ovary and maximum and mean cross-sectional ovary
areas were measured. Oocyte number or sizes may also be
measured
in situ
. MacInnis and Corkum (2000) examined
nests of the invasive round goby (
Neogobius melanosto-
mus
) through video recordings. The area of each egg
mass or area covered by egg scars was subsequently mea-
sured using IA. Analysing the data in association with
conventional methods (e.g. egg counting by hand and
ovarian weighting) allowed the authors to conclude that
the reproductive strategy of round gobies combined with
its aggressive behavior may favour the species expansion
throughout the Great Lakes.
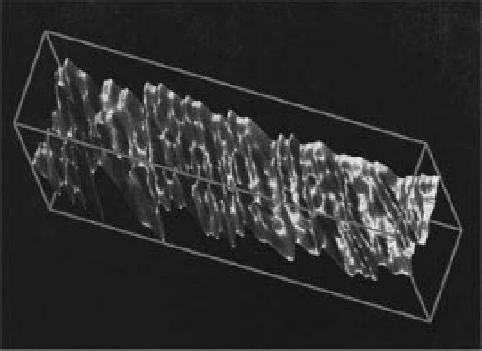
Figure 17.7
Visualisation of fine growth increments (seasonal
and monthly increments) on a molluscan shell. Reprinted from
Computers & Geosciences, 25(8), Toubin, M. et al., Multi-scale
analysis of shell growth increments using wavelet transform,
pp. 877-885, Copyright 1999, with permission from Elsevier.
To extract the information from variations in shell
topography, Toubin et al. (1999) used a technique involv-
ing multi-scale analysis of the shell topography after a
wavelet transform. An optical system, based on laser tri-
angulation, mapped the shell surface (Figure 17.7). A
multiscale representation allowed distinctions between
growth increments of various orders (seasonal, monthly,
daily), which were related to bivalve ontogenesis and
environmental stress.
Furthermore, the analysis of molluscan shell growth
may allow the reconstruction of environmental condi-
tions
a posteriori
.Schone et al. (2004) inferred summer
air temperatures for each year over the period 1777-1993
from studying variations in annual shell growth of the
freshwater pearl mussel
Margritifera margritifera
(using
both live and collection specimens). Up to 55% in the
variability of annual shell growth was explained by tem-
perature changes. Such models can be used to test and
verify other air temperature proxies and thus may help
improve climate models. However, Dunca et al. (2005)
underlined that shell growth does not co-vary with
summer temperatures at polluted sites, suggesting that
care is required when designing an appropriate sam-
pling strategy when molluscan shells are used for climate
reconstructions.
Ageing based on fish otoliths is often a subjective activ-
ity, based on experience (see Abecasis et al., 2007 for
a comparison of aging fish from scales and otoliths).
Evidently, although it is possible, it is more difficult
to determine fish age at small time scales (e.g. age in
days
vs
age in years). Over the last 35 years (following
17.2.2.2 Growth
Population studies depend upon correct age and growth
estimates. There are a few ways to assess individual
growth: by (1) analysing differential body lengths, (2)
studying anatomical structures like shells, scales or
otoliths and (3) quantifying chemical substances that
accumulate within the body with age (e.g. lipofuscin).
Monitoring the growth of live fish without manipulating
specimens is a difficult task. Tillett et al. (2000) described
an underwater stereo IA technique that offered the poten-
tial for estimating key dimensions of free-swimming fish.
Comparing automatic measurements of fish dimensions
with manual measurements demonstrated an average
length error estimation of 5%.
In most cases, periodic growth increments have been
measured to estimate the age. Tree rings are the archetypal
ageing structure. In freshwater animals, growth incre-
ments of various anatomical structures (such as bivalve
shells, tortoise scute, fish scales, vertebrae, fin rays, oper-
cula and otoliths) are used to estimate age and reconstruct
growth rate. In molluscans, shell increments contain
information related to the evolution of the environment
in which the organism grew during its biomineralisation.
Search WWH ::

Custom Search