Information Technology Reference
In-Depth Information
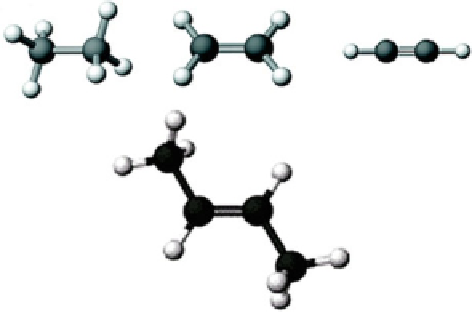
Fig. 3.16 Main structural
forms of carbon compounds
the carbon itself and the two atoms associated with it—lie on a straight line. Since
in the vast majority of organic compounds the valence of carbon is equal to four,
trigonal states appear in the molecules with double carbon-carbon bonds, while the
linear states in the molecules with triple bonds. Moreover, different structural
elements can be combined within the same molecule.
These structural features of the molecules of carbon compounds can be
explained based on the properties of their electronic structure. Today, the geometric
configuration of the nuclei of relatively large organic molecules can be determined
with experimental accuracy by solving the Schr¨dinger equation, but this is a
tedious and expensive approach. Therefore, already in the 1930s of the last century,
when computational quantum-mechanical methods were still in their infancy, the
famous chemist Linus Pauling proposed a theoretical semiempirical method. He
suggested an explanation of the experimentally determined variants of bond posi-
tions of the carbon atom based on the principle of hybridization of atomic orbitals.
As is known, the valence electrons of the carbon atom are located at the 2s and
2p orbitals. The 2s orbitals have spherical symmetry, and the 2p orbitals are located
in the space perpendicular to each other. Since linear combinations of the solutions
of the Schr¨dinger equation also represent its solutions, Pauling introduced the
concept of hybrid orbitals—linear combinations of s and p orbitals of the carbon
atom. If one imposes symmetry constraints on these combinations, it turns out that
they correctly describe the spatial arrangement of carbon atom bonds (Figs.
3.17
,
3.18
, and
3.19
). In the case of tetrahedral symmetry, for example, upon turning the
hybrid orbitals by 120
with respect to one of them, they will coincide with each
other. Taking into account the symmetry principles it could be shown that in the
case of tetrahedral symmetry all s and p atomic orbitals of carbon are present in all
four hybrid orbitals. This kind of hybridization is called sp
3
hybridization. At the
same time, in accordance with symmetry requirements the trigonal (sp
2
) and linear
(sp) hybrid orbitals must consist of an s and two p and an s and one p orbitals,
respectively. Consequently, there remain orbitals that are not part of hybrid
orbitals—one p orbital in the case of sp
2
hybridization and two in the case of sp