Information Technology Reference
In-Depth Information
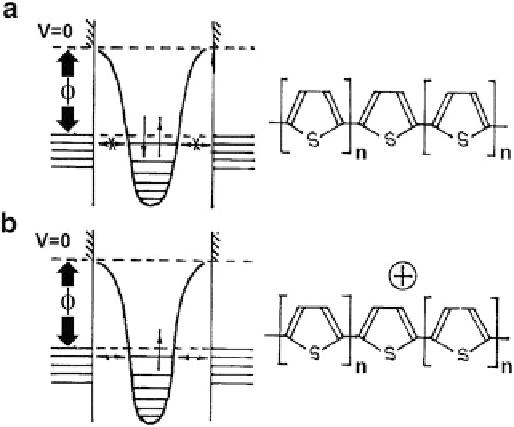
Fig. 3.9 Structure of the electronic levels and the chemical formula of the Aviram molecular
switch in the nonconducting (a) and conducting (b) form
ensemble—an ordered molecular film. This is no accident. In essence, rather than
describing the passage of electric current through a molecule, the model of Aviram
and Ratner describes single-electron transfer between the fragments of the mole-
cule. This immediately casts doubt on the suitability of systems of this type for the
creation of molecular switching elements. Because the molecule of Aviram and
Ratner is a quantum object, there is only a certain probability that the molecule will
switch to the conducting state under the influence of the voltage applied to the
electrodes. In contrast, a switching element should always respond to the applied
stimulus.
Therefore, in 1988 Aviram proposed another version of the molecular complex
designed, as he defined it, for “memory, logic, and amplification.” The structure of
this complex was based on extensive molecular fragments (at least ~50
long). It
was known that the electronic structure of these fragments is similar to the band
structure and that they can be in two states—conducting and nonconducting,
moving from one state to another during reductive-oxidative processes. The
highest filled molecular orbital of the nonconducting form is situated below the
Fermi level of the metal (Fig.
3.9
). Therefore, if such a molecule is placed between
two electrodes, the applied voltage does not cause the passage of electrons through
the molecular fragment. However, the current will pass if one electron is removed
from the top orbital of the fragment, turning it into a positively charged ion. The
molecular switch proposed by Aviram is shown in Fig.
3.10
. In this complex
conductive and nonconductive parts are perpendicular to each other and are
connected by a nonconducting hydrocarbon fragment.
Å