Information Technology Reference
In-Depth Information
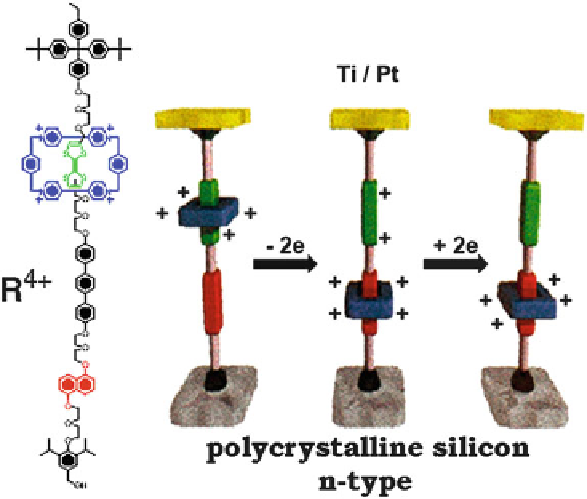
Fig. 3.39 Switch based on the rotaxane molecule
In the molecule of [2] rotaxane R, selected by the Hewlett-Packard, University
of California group, an electron donor group—tetrathiafulvalene—is built into the
main chain, and the cycle moving along the chain is constituted by a tetracation
nitric group, an electron acceptor. A conformational rearrangement of the molecule
occurs (Fig.
3.39
) when the donor loses an electron. As a result, an electrostatic
repulsion of the cycle from the donor group occurs with subsequent rearrangement
of the molecular structure of the rotaxane. The peculiarity of this molecule is its
amphiphilic character.
This term implies that the molecule is a long molecular chain with pronounced
hydrophobic properties. A hydrophilic group is located at one end of this chain.
This specific structure of amphiphilic molecules underlies the Langmuir-Blodgett
method for forming structurally ordered monomolecular films.
This method involves pouring the solution of amphiphilic compounds on water
surface using the device called Langmuir-Blodgett trough (Fig.
3.40
). Due to
hydrophobicity of the chains, molecules position themselves on the water surface
in an arbitrary manner, although the terminal hydrophilic groups actively interact
with water. A barrier swept across the surface compresses the film, with hydrophilic
groups remaining in the water and the hydrophobic tails lining up perpendicular to
the surface. If a solid substrate is introduced into the film being compressed and is
gradually extracted from the trough upon film contraction, the monomolecular layer
of the amphiphilic substance gets transferred to the substrate. Repeating this
process many times, one can obtain multimolecular layers.