Geology Reference
In-Depth Information
Further reading
Albarède, F. (2009)
Geochemistry, an Introduction
, 2nd edition.
Cambridge:Cambridge University Press.
Atkins, P.W. and de Paula, J. (2009)
Elements of Physical
Chemistry
, 5th edition. Oxford: Oxford University Press.
Barrett, J. (2003)
Inorganic Chemistry in Aqueous Solution
.
Cambridge: Royal Society of Chemistry.
Drever, J.I. (1997)
Geochemistry of Natural Waters
, 3rd edition.
Upper Saddle River, NJ: Prentice Hall.
Edmond, J.M. and von Damm, K. (1983) Hot springs on the
ocean floor.
Scientific American
248
(April), 78-93.
Krauskopf, K.B. and Bird, D.K. (1995)
Introduction to
Geochemistry
, 3rd edition. New York: McGraw-Hill.
Libes, S.M. (2009)
An Introduction to Marine Biogeochemistry
,
2nd edition. San Diego:Academic Press.
Rankin, A. (2005) Fluid inclusions. In
Encyclopedia of Geology
(eds Selley, R.C., Cocks, L.R.M. and Plimer, I.R.) p. 253-60.
Amsterdam: Elsevier.
Smedley, P.L. and Kinniburgh, D.G. (2002) A review of the
source, behaviour and distribution of arsenic in natural
waters.
Applied Geochemistry 17
, 517-68
UNICEF (2008) Arsenic mitigation in Bangladesh. Available
Walther, J.V. (2008)
Essentials of Geochemistry
, 2nd edition.
Sudbury, MA: Jones and Bartlett Publishers.
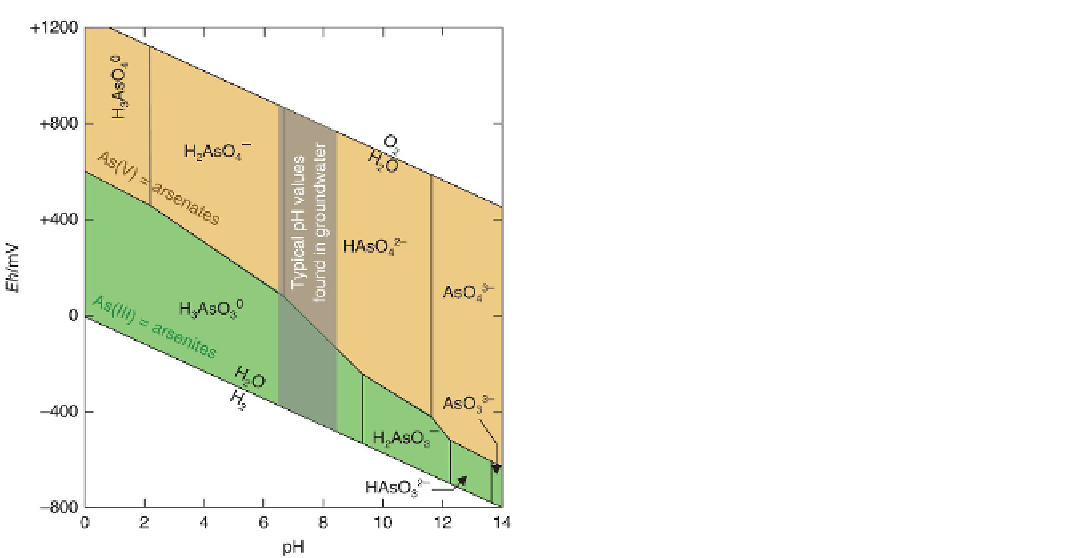
Figure 4.2
Eh
-pH diagram showing the stability ranges of
dissolved forms of arsenic. Arsenic exists in aqueous solution
in one of two
oxidation states
(see Box 4.7): As(III) in the
lower, light orange field, and As(V) in the darker field above.
In each field, dissolved arsenic occurs as a number of pH-
dependent
oxy-anions
, some of which incorporate hydrogen
(cf. bicarbonate HCO
3
−
). The darker vertical band illustrates
the limited range of pH values characteristic of most ground-
water samples. (Source: Adapted from Smedley & Kinniburgh
(2002). Reproduced with permission of Elsevier.)
Exercises
4.1
Calculate the solubility (in mol kg
−1
) of BaSO
4
at
25 °C in:
(a) pure water;
(b) water containing 10
3
mol kg
−1
dissolved CaSO
4
.
4.2
Calculate how many grams of CaF
2
will dissolve in
1 kg of pure water at 25 °C. (Solubility product of
CaF
2
25 °C = 10
−10.4
; Ca = 40; F = 19.)
4.3
Calculate the activity of carbonic acid in rainwater
that has equilibrated with air:
(a) with a nominal pre-industrial
P
CO
air
2
(Nickson
et al
., 2000). When reducing conditions
develop in the aquifers after burial, these iron coatings
dissolve and liberate Fe and As into the groundwater.
It is estimated that 57 million people, mostly in SE
Bangladesh, are drinking water with As levels higher
than the current recommended
WHO
safe upper limit
of 10 μg dm
−3
; 30 million of these even depend on water
with As levels above 50 μg dm
−3
(Smedley and
Kinniburgh, 2002). In the short term, partial decontam-
ination is possible by thoroughly aerating drinking
waters drawn from the contaminated wells: this causes
re-oxidation and precipitation of the dissolved Fe and
coprecipitation of some of the As from solution.
Still deeper wells (>150 m in depth) yield water that
has acceptably low As levels and may provide a longer-
term answer to SE Bangladesh's chronic water supply
problems.
=
.
Pa,
28 0
(b) with the actual mean value of
P
CO
air
2
=
.
Pa
31 5
measured in 1960, and
(c) with the actual mean value of
P
CO
air
2
=
.
Pa
39 1
measured in 2012.
12
Calculate the pH values of these solutions (consider-
ing only reaction 4.21) and estimate the change in
rainwater pH over the last 50 years. (Equilibrium
constant
K
HCO
2
(reaction 4.20) = 0.31 × 10
−6
mol kg
−1
3
Pa
−1
at 25 °C.)


Search WWH ::

Custom Search