Geology Reference
In-Depth Information
(b)
(a)
+1200
+1200
Increasing acidity
= more protons
+800
Acid
mine
waters
+800
Cu
2+
Rain
Streams
Oceans
Peat
& bog
waters
+400
+400
CuCl
3
2-
Shallow
groundwater
0
0
Connate
waters
-400
-400
Dissolved Cu species
Crystalline Cu minerals
-800
-800
0
2
4
6
8
10
12
14
0
2
4
6
8
10
12
14
pH
pH
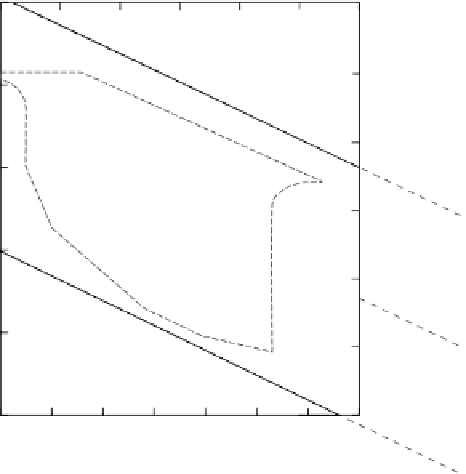
Figure 4.1
Eh
-pH diagrams, showing (a) approximate stability fields of copper minerals and dissolved spacies (shaded area)
in equilibrium with water, S
2−
, Cl
−
and CO
2
2−
(simplified from Rose (1976, 1989) using malachite data from Vink (1986) and
Garrels and Christ (1965)); (b) approximate
Eh
-pH ranges of some natural aquatic environments. Dots and the enclosing
dashed line delimit the range of water analyses observed in nature, from the compilation by Baas Becking
et al.
(1960).
Box 4.8 Drinking water quality
taminants in their public water supplies. When contami-
nant concentrations are found to exceed prescribed limits
(e.g. WHO, 2011), a utility must shut off the affected
source from the supply network, or blend in water from
other sources to bring the concentrations down below the
prescribed limit.
It is good practice to monitor a wider range of water
quality indicators of concern to the comsumer, including
physical attributes like transparency, taste, odour and col-
our, as well as technical chemical parameters such as pH,
hardness, dissolved oxygen content, and
chemical oxygen
demand
(COD - a measure of the total oxidizable organic
compounds present).
What factors determine whether water is safe to drink?
Four categories of water contamination pose a potential
threat to human health:
●
Microbiological contaminants, including viruses and
bacteria (e.g. fecal coliform bacteria caused by inad-
equate sewage treatment of discharges upstream).
●
Inorganic chemical solutes
11
such as nitrate and phos-
phate (which are usually traceable to fertilizer use) and
heavy metals.
●
Organic chemical contaminants such as industrial sol-
vents, pesticides and pharmaceuticals.
●
Radiological contaminants such as radon (Box 3.3) and
radioiodine (
131
I).
In developed countries, water utilities bear responsibility
for regularly monitoring the concentrations of such con-
11
Some inorganic solutes occur naturally and may, like Ca
2+
and Mg
2+
, be beneficial to health.










Search WWH ::

Custom Search