Geology Reference
In-Depth Information
P
LIQUID
Conditions of
entrapment
Inclusion cools
within host crystal along
constant-volume
P
-
T
path
A
CP
Bubble nucleates
T
A
Crystal nucleates
B
SOLID
B
1
D
T
B
R
D
1
TP
VAPOUR
T
Owing to the large refractive
index difference, the bubble
has much higher optical
relief than the inclusion
or daughter crystal.
Figure 4.6.2
The
P
-
T
path of fluid inclusion formation.
aerated are oxidizing and lie in the upper, 'oxic' part of
the 'water window'. Ferrous minerals coexisting with
them (Box 4.7) are eventually oxidized to red ferric min-
erals such as hematite (Fe
2
O
3
) or goethite (hydrated
Fe
2
O
3
), and iron-bearing rocks like some shales and
sandstones therefore weather a reddish colour. Stagnant
or waterlogged environments - particularly those rich
in organic matter - tend to be strongly reducing (lying in
the lower 'anoxic' part of the water window). Weathering
in these conditions, found below the water table, pro-
duces grey or green surfaces characteristic of ferrous-
iron silicate minerals. Magnetite (Fe
3
O
4
= FeO.Fe
2
O
3
) and
sulfide minerals are also stable in such environments.
Eh
-pH diagrams serve a similar purpose for the sed-
imentary geochemist as
P-T
or
T-X
phase diagrams
(Chapter 2) do for 'hard-rock' petrologists, helping us
to reconstruct from the minerals contained in an actual
rock the conditions under which it formed. Many



























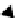

Search WWH ::

Custom Search