Geology Reference
In-Depth Information
Box 3.4 What activation energy means on the atomic scale
In a chemical reaction (Figure 3.3):
constitutes the activation energy
E
a
(Figures 3.3 and
3.4.1c). the whole reaction from aB to BC can be visual-
ized by considering the energy-distance curves of both
molecules, Figures 3.4.1a and b, 'back to back' as in
Figure 3.4.1c. Note that the aB bond need not be com-
pletely broken before the BC molecule can begin to form.
the explanation of the activation energy in reactions
between ionic compounds is slightly different, but is still
associated with the need to disrupt one arrangement of
atoms or ions before another more stable arrangement
can be adopted.
AB CABC
+→+
the established a-B bond must be weakened (stretched)
before a new bond (B-C) can begin to form. the energetics
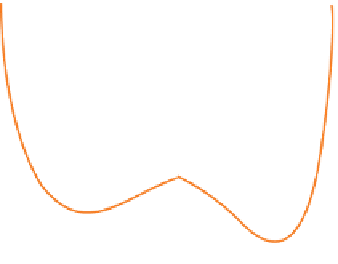
of the a-B bond are shown in Figure 3.4.1a. the process
begins with aB in its most stable configuration (inter-
nuclear distance =
r
AB
−
0
). energy is required to stretch the
a-B bond to the stage when formation of the B-C bond
becomes an equally probably outcome (i.e. sufficient to
form the activated complex a
…
B
…
C); this energy input
(a)
(b)
B
C
A
B
E
r
B-C
r
A-B
r
A-B
r
B-C
0
r
A-B
°
r
B-C
°
r
A-B
r
B-C
(c)
E
0
E
a
A
...
B
...
C
A-B
B-C
r
A-B
r
B-C
Figure 3.4.1
(a) and (b). Bond energy versus nuclear separation
r
a-B
and
r
a-B
for molecules aB and BC. (c) Bond energy
and activation complex in the transition from aB + C to a + BC.

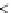
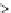

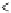



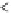










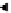
















Search WWH ::

Custom Search