Geology Reference
In-Depth Information
Box 2.3 Tie-lines and the Lever Rule
In
T-X
,
P-X
or
P-T-X
diagrams, tie-lines link together the
compositions of two different phases that can coexist in
equilibrium under specific conditions. Any composition
lying between the ends of a tie-line must therefore repre-
sent a physical
mixture
of the two phases. From the posi-
tion of that point on the tie-line, one can work out the
relative proportions of the two phases in the mixture.
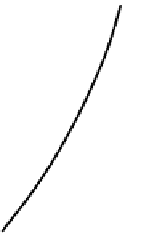
Figure 2.3.1a shows part of a phase diagram in which
complete solid solution exists between two compounds, A
and B (cf. Figure 2.5). The tie line
c-d
depicts equilibrium
at temperature
T
1
between a melt of composition
c
on the
liquidus, and a solid solution of composition
d
on the soli-
dus; both
c
and
d
are expressed in mass % B. Composition
x
lies in the
two-phase field
between
c
and
d
, and must
signify a physical mixture of these two distinct phases. Let
C
and
D
represent the mass fractions (
i.e. C
+
D
= 1.00) in
which
c
and
d
are mixed to form
x
. We can then express
the composition of
x
as a weighted average of
c
and
d
:
Substituting
D
= 1 -
C
into Equation 2.3.1, we can show in
a similar fashion that:
C
xd
cd
−
−
dx
dc
−
−
(
)
=
=
thechangeinsigncancelsout
The mass ratio in which
c
and
d
are present in
x
is there-
fore given by:
C
D
dx
dc
−
−
xc
dc
−
−
dx
xc
−
−
(
)
=
=
aftercancelling denominators
(
)
=
(
)
Inother words:
CxcDdx
−
−
(2.3.2)
This useful equation is known as the Lever Rule, since
it can also be applied to the 'lever effect' of the old-
fashioned beam-balance (Figure 2.3.1b), in which the
weight of a body
C
is inversely proportional to the dis-
tance from the fulcrum (
c-x
) at which it balances an
opposing weight
D
:
weight of C
weight ofD
=
xd
cx
−
−
(2.3.1)
xCcDd
=+
Since
C
= 1 -
D
, this can be rewritten:
Qualitatively, the closer the composition of a mixture
plots (in composition space) to one of its constituents,
the greater the percentage of that constituent in the
mixture.
=
(
)
+= +
x
1-
D cDdcDc Dd
-
Therefore
x - c
=
D
(
d - c
)
leading to
D
xc
dc
−
−
=
(a)
Melt field
x
c
T
1
d
Solid-solution
(crystal) field
Two-phase field
Composition
A
B
(b)
C
D
c
d
x
Figure 2.3.1
(a) Part of a phase diagram similar to Figure 2.5 to illustrate the Lever Rule. (b) The analogous geometry
of the beam-balance.

























Search WWH ::

Custom Search