Geology Reference
In-Depth Information
Box 2.2 Other one-component phase diagrams
Graphite-diamond
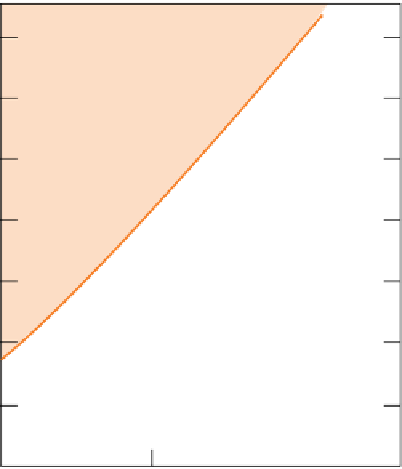
The phase relations between the two main crystalline
forms of carbon (Chapter 8) are shown in Figure 2.2.1a.
Note the very high pressure (in excess of 20 × 10
8
Pa)
required to stabilize diamond at relevant temperatures.
For this reason, diamond can only form naturally deep
inside the Earth's mantle (an equivalent depth scale is
shown at the right-hand side). Moreover, the minimum
pressure increases with temperature, so still higher
pressures are necessary to stabilize diamond in the hot
interior of the Earth than would be the case at room tem-
perature. The curve marked 'geotherm' shows how tem-
perature increases with depth beneath ancient continental
shields (the geological setting where diamond-bearing
kimberlites are found). From the point at which the geo-
therm enters the diamond stability field, it is clear that
diamonds can only be formed at depths greater than about
120 km (≈40 × 10
8
Pa).
Each stability field in Figure 2.2.1a is divariant, and the
boundary between them is univariant. Because there are
(a)
(b)
H
2
O
P
CO
2
70
210
60
180
Diamond
Liquid
Solid
50
150
P
A
Gas
T
40
120
(not to scale)
30
90
Graphite
Supercritical
fluid
C
Liquid water
221
20
60
Ice(I)*
T
b
T
m
P
A
10
30
0.06
Vapour
T
0
0
0
1000
2000
0.008
100
374
T/°C
T
/°C
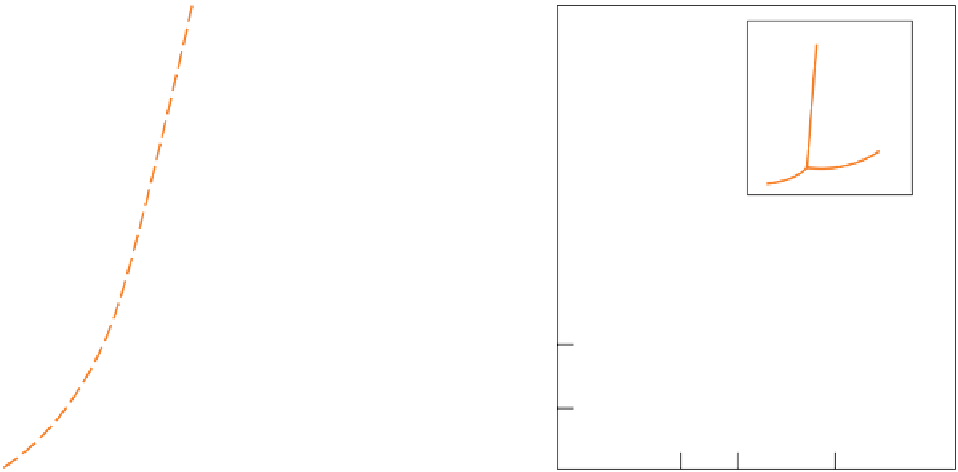
Figure 2.2.1
(a) The
P-T
phase diagram for the two main forms of elemental carbon, graphite and diamond. The curve
marked 'geotherm' shows how temperature increases with depth beneath ancient continental cratons. (b) The
P-T
phase diagram for the system h
2
O (ice-water-vapour). The inset sketches the corresponding phase diagram for CO
2
.
would consist of albite + jadeite, again a divariant
assemblage. The only way to form the one-phase
assemblage - albite alone - is to combine jadeite and
quartz in exactly equal molecular proportions, so
that no quartz or jadeite is left over. In other words,
to generate just albite in passing from X to Z we must
control not only
P
and
T
but also a
compositional prop-
erty
of the system - the NaA1Si
2
O
6
: SiO
2
ratio. This
compositional requirement is the unsuspected third
degree of freedom whose existence the Phase Rule
has uncovered.
P-T
diagrams provide useful vehicles for portraying
metamorphic conditions in the crust (Yardley, 1989)
and for showing the way in which they change with
time (so-called
P-T-t
paths) during mountain building
episodes (Barker, 1998; Best, 2002).
P-T
diagrams are






Search WWH ::

Custom Search