Geology Reference
In-Depth Information
(a)
GOE
'red beds'
?
BIFs
150
Present level of O
2
100
Archaean
Proterozoic
50
pP
mP
nP
Phan
0
4
3
2
1
0
Age/Ga
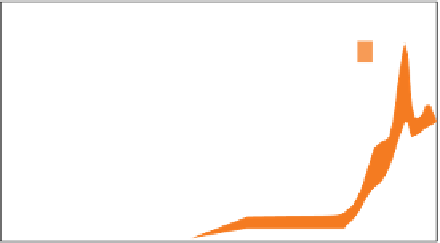
Figure 11.8
(a) Cartoon illustrating, from left to right, the stepwise evolution of atmospheric oxygen content from the
Archaean to the present as envisaged by Holland (2006), expressed as volume % of the present atmospheric level (PAL);
the thickness of the band reflects estimated uncertainty. The subdivisions of the Proterozoic eon are abbreviated as pP
(Palaeoproterozoic), mP (Mesoproterozoic), nP (Neoproterozoic). Phan = Phanerozoic. 'GOE' shows the duration of the Great
Oxidation Event referred to in Chapter 10 (Figure 10.13). The horizontal orange bars indicate the periods over which iron
sedimentation was dominated respectively by banded iron formation (BIF) and by red beds; the darker BIF bars represent
the main episodes of BIF deposition identified by Isley and Abbott (1999). The brief resumption of BIF deposition in the
Neoproterozoic correlates with three 'Snowball Earth' episodes. (b) Field picture of early Archaean banded iron formation,
Isua, West Greenland. (Sources: Own based (a) on Holland 2007; Reproduced with permission of GEUS).
the δ
13
C record (Figure 10.13). Curiously, there is indir-
ect evidence suggesting this was also a period of rap-
idly
increasing
atmospheric O
2
(Figure 11.8a; Frei
et al.
,
2009), implying conditions quite different to those that
promoted BIF deposition in Archaean times. This par-
adox can be resolved if the Neoproterozoic ice sheets
largely isolated the oceans from the atmosphere, so
that iron introduced into the oceans from seafloor
hydrothermal vents could accumulate as dissolved
ferrous iron until glacial retreat allowed oxygen
exchange and precipitation of the dissolved iron. In
any event, the Neoproterozoic glaciations must again
reflect large-scale drawdown of atmospheric CO
2
, but
whether this had an entirely biological origin (e.g. col-
onization of continents by photosynthetic biota) or
was somehow related to profound tectonic changes
then taking place (notably the break-up of the Rodinia
supercontinent) remains unclear.
The manner in which life adapted to the prevalence
of oxidizing conditions at the Earth's surface, putting
oxygen to good use to generate energy, is a fascinating
story explored in the topic by Lenton and Watson
(2011). The earliest life-forms evidently developed on
an abundant supply of organic nutrients that could
exist stably in the oxygen-free primordial atmosphere.
Such organisms would not be able to develop in the
Earth's present atmosphere, where oxidation would
rapidly destroy their simple molecular foodstuffs, any
more than we could survive in oxygen-free conditions.
Life, by introducing free oxygen into the atmosphere
and sustaining it there for more than 2 billion years
(when the residence time of oxygen in the atmosphere
is only a few thousand years), has burned the environ-
mental boat by which it first came into being.
Yet life has also transformed the Earth into the toler-
able planet to live on that it currently is. All of the oxy-
gen in the atmosphere has been manufactured by
photosynthetic organisms from carbon dioxide, and by
lowering atmospheric CO
2
levels such organisms have
thereby turned down the heat in the Earth's 'green-
house' to a much lower level than operates on Venus,
whose atmospheric greenhouse maintains the surface
temperature at a searing 470 °C. This mechanism for
removing CO
2
from the Earth's atmosphere is essen-
tially a reversible equilibrium, balancing oxidized car-
bon in the air against reduced carbon in the biosphere.
However, a small proportion of reduced biosphere car-
bon has also became fixed in the crust in the form of









Search WWH ::

Custom Search