Geology Reference
In-Depth Information
Mass-independent fractionation of sulfur isotopes
Samples younger than 2200 Ma (darkest circles) have
Δ
33
S values very close to zero, indicating normal
mass-dependent fractionation, whereas samples
older than 2200 Ma (intermediate circles) deviate
to positive values up to 2, and Archaean samples
(lightest circles) vary erratically over a much wider
range. Prior to 2450 Ma, when atmospheric oxygen
levels were negligible, no stratospheric ozone (O
3
)
layer could form, and therefore - unlike today - the
atmosphere and the Earth's surface were exposed to
the full force of incoming solar UV radiation. Under
such conditions, photochemical reactions fractionate
sulfur isotopes in a more chaotic
mass-independent
manner in which Equation 10.12 no longer holds. The
transition from a
mass-independent
(kinetic)
fractionation
of sulfur
('MIF-S') signal in Archaean times to mass-
dependent (equilibrium) fractionation (Δ
33
S ~ 0.0) in
younger samples provides further evidence for the
first emergence of significant atmospheric oxygen
(some of which formed ozone) around the close of
the Archaean eon (cf. Figure 11.8).
Since oxygen and sulfur both have more than two
stable isotopes, we can in principle formulate several
δ values (δ
17
O, δ
18
O, δ
33
S, δ
34
S, δ
36
S), although normally
only those involving the two more abundant isotopes
of each element (δ
18
O, δ
34
S) are measured. Most natural
processes fractionate oxygen and sulfur isotopes in a
predictable,
mass-dependent
way. In other words, we
would expect the δ
17
O value of a sample to be roughly
half of its δ
18
O, since the mass difference relative to
16
O
is halved (17 − 16 compared to 18 − 16) and the degree
of fractionation follows suit. This expectation is borne
out by measurements on a range of meteoritic and
terrestrial materials:
δ
17
O
≈×
052
.
δ
18
O
(10.11)
δ
33
S
≈
0 515
.
×
δ
34
S
(10.12)
Do circumstances occur in which this relationship
breaks down? One conspicuous example where it has
is illustrated in Figure 10.14, which shows - in a time
plot similar to Figure 10.13 - how the sulfur isotope
composition of sedimentary sulfides and sulfates has
varied throughout Earth history. The
y
-axis shows
the departure of measured δ
33
S from the mass-
dependent value expected from Equation 10.12.
Transition metal stable isotopes
The remarkable capacity of living organisms to frac-
tionate isotopes is not confined to low-mass isotope
systems like
13
C/
12
C. Figure 10.15 shows how
66
Zn/
64
Zn
12
> 2450 Ma
2200-2450 Ma
10
< 2200 Ma
8
MIF-S caused by intense
solar UV owing to lack of
protective ozone layer
6
4
Ozone layer in oxygen-bearing
atmosphere prevents MIF-S
2
0
4000
3500
3000
2500
2000
1500
1000
500
0
-2
Age (millions of years)
-4
Figure 10.14
Plot of
Δ
33
S = δ
33
S
meas
− 0.515 × δ
34
S
meas
(cf. Equation 10.13) in sedimentary sulfides and sulfates as a function of age
from 4 Ga to the present. (Source: Reproduced with permission of David Johnston of Harvard University.)












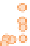



























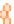































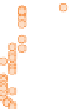



















Search WWH ::

Custom Search