Geology Reference
In-Depth Information
δ
18
O can also be used to measure much higher
temperatures of equilibration between minerals and
igneous melts, although with lower precision.
Depth in Vostok borehole/m
500
1000
1500
2000
-53
-420
(a)
-55
Carbon stable isotopes - detecting signs
of ancient life
-440
-57
14
-59
Carbon has two stable isotopes,
12
C and
13
C (Figure 10.9).
Owing to the 8% difference in atomic mass between
them, geochemical reactions discriminate to a small
extent between the two isotopes. Dissolved carbon
dioxide and carbonate sediments in the oceans contain
a higher
13
C/
12
C isotope ratio (by 5-10‰) than atmos-
pheric CO
2
(Figure 10.13).
Organic matter, on the other hand, is strongly
depleted in
13
C, leading to δ
13
C values - in marine
phytoplankton, for instance - that are about 20‰
lower than atmospheric carbon dioxide. This reflects a
remarkable isotopic fractionation during photosynth-
esis that can be traced to a key enzyme known as
Rubisco,
10
the most abundant protein in green leaves. It
is Rubisco that fixes the atmospheric CO
2
absorbed
during photosynthesis, and in doing so it exerts a
marked preference for
12
C. This fact makes δ
13
C an
invaluable tracer for photosynthesis in the geological
record, for instance in detecting the beginnings of
photosynthetic life in ancient sedimentary successions.
This can be illustrated by reference to Figure 10.13.
Carbon from atmospheric CO
2
is locked up in sedi-
mentary rocks in two forms:
-61
12
-470
-63
10
-65
-490
(b)
8
Last glaciation
6
0
25
50
75
100
125
150
Age/ka
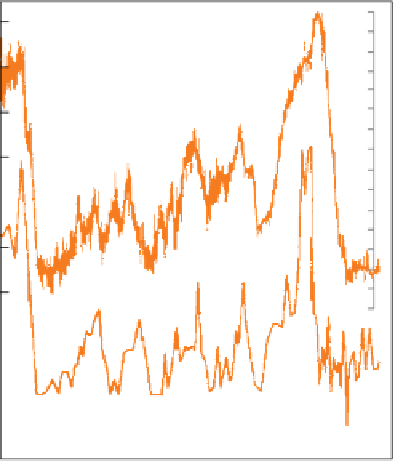
Figure 10.12
(a) Variation in mean annual surface tempera-
ture at Vostok Station in East Antarctica over the past
160,000 years (left-hand scale), based on δD measurements
on Vostok deep ice cores; the orange scale on the right
indicates the corresponding δD values (b) Variation over the
same period of summer sea-surface temperature (scale on
far right), based on statistical analysis of radiolarian
assemblages in borehole RC11-120 in the southern Indian
Ocean. (Source: Adapted from Jouzel
et al.
, 1987.
Reproduced with permission of Nature Publishing Group;
Data from Martinson
et al.,
1988.)
precipitated, and systematic sampling of ice cores
drilled from ice caps allows an isotopic record of
Quaternary climate variation to be assembled. The
age-calibrated Vostok ice cores from eastern Antarctica
have been shown to provide a continuous record of
precipitation extending over the past 420,000 years
(Petit
et al.,
1999). Figure 10.12a shows how δD values
and calculated mean air temperatures at Vostok have
varied over the last 160,000 years. A trace of sea-sur-
face temperatures in the subpolar Indian Ocean (based
on diatom populations) over the same period is shown
for comparison (Figure 10.12b); although covering
very different latitudes and temperature ranges, the
two traces show the same glacial-interglacial climate
variations. Both are important isotopic proxies that
contribute to our knowledge of natural global climate
change.
• inorganic carbonate which - although it resides in
the shells of living creatures - is broadly in isotopic
equilibrium with atmospheric CO
2
with δ
13
C values
straddling zero (Figure 10.13);
• reduced'organic' carbon (in forms such as coal and
oil) derived from decomposed living soft tissue.
Since biological carbon - even in animals - is ult-
imately derived from the atmosphere by photosyn-
thesis, all organic carbon in sediments carries the
negative δ
13
C 'Rubisco signature'.
Over much of the geological record, the marine carb-
onate δ
13
C values shown in Figure 10.13 maintain
10
Abbreviation of the enzyme's full name: Ribulose-1,5-
bisphosphate carboxylase oxygenase.


Search WWH ::

Custom Search