Geology Reference
In-Depth Information
(a)
Crystal of
potassium mineral
(b)
(c)
t
= 0
40
K nuclei
40
Ar nuclei
t
=
t
1
t
=
t
2
40
Ca nuclei
(d)
(e)
(f)
t
= 0
t
=
t
1
t
=
t
2
40
Ca
40
K
40
Ar
40
Ca
40
K
40
Ar
40
Ca
40
K
40
Ar
Radioactive
40
K
Radiogenic
40
Ar measured for dating
Radiogenic
40
Ca
Non-radiogenic
40
Ca originally present in the crystal
Figure 10.3
Cartoons and bar charts illustrating the decay of
40
K to
40
Ar and
40
Ca in a potassium-bearing crystal.
The complexity of Equation 10.1 is a consequence of
the branched decay scheme of
40
K.
Various K-rich minerals separated from plutonic or
metamorphic rocks may be used for K-Ar dating,
including biotite, muscovite and hornblende. For dating
volcanic rocks, feldspars (sanidine, anorthoclase, plag-
ioclase) and whole-rock samples are often used as well.
A K-rich mineral is likely to contain a trace of calcium
as well, so
40
Ca will be present in it at the outset
(Figure 10.3a,d).
1
Distinguishing a small contribution of
radiogenic
40
Ca (the product of
in situ β
-
-decay of
40
K) from
the non-radiogenic component of
40
Ca already present
(Figure 10.3a,d) is problematic, and so the
40
Ca branch of
the
40
K decay scheme is not used in geochronology.
K-Ar dating, although straightforward to under-
stand, is prone to
systematic errors
that are hard to
quantify, and so it is rarely used today. It has been super-
seded by the more reliable
40
Ar/
39
Ar geochronometer
(Box 10.2).
Rb-Sr geochronology
The initial absence of
40
Ar from the potassium-bearing
mineral or rock being dated makes the K-Ar and Ar-
Ar dating techniques relatively straightforward, allow-
ing an age to be determined from a single rock sample.
For most geochronometers, however, an unknown
amount of the daughter isotope occurs naturally in the
sample from the outset. In such cases
several
cogenetic
samples
need to be analysed if we are to eliminate this
unknown factor and secure an accurate age.
The Rb-Sr isotope system (introduced in Figure 10.1)
illustrates the principles. The way in which Sr isotopic
composition varies with time is sketched out in
Figure 10.4. The vertical axis represents the amount of
40
Ca is the most abundant isotope of calcium, making up 97%
of the element.
1















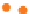
















































































Search WWH ::

Custom Search