Geology Reference
In-Depth Information
Box 9.10 Transition metals and the colour of minerals
d-orbitals project a long way from the nucleus and are
highly directional (Figure 5.5). d energy levels are therefore
sensitive to the positions of surrounding ligands.
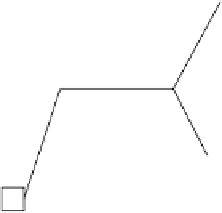
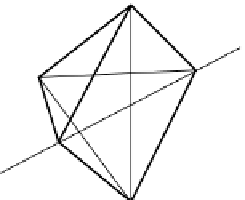
Figure 9.10.1 a depicts a transition metal in a regular
octahedral site in a crystal, surrounded by six equidistant
anions, which one can imagine positioned on the refer-
ence axes used for describing orbital geometry (Chapter 5).
the co-ordination structure is shown cut in half, to clarify
the geometry, as is the co-ordination polyhedron sketched
in the cartoon (b). Because of the potential repulsion
between these anions and electron density in the trans-
ition metal d orbitals, the most stable d electrons will be
those in orbitals that interfere least with the octahedrally
positioned ligand anions.
placing a transition metal in octahedral co-ordination
brings two changes in d energy levels. the mean energy
increases, due to the overall repulsion by the anion field.
Secondly, the energy levels are split. Orbitals like the d
yz
example shown, whose lobes point at the edges of the co-
ordination polyhedron (between the ligands), have a lower
energy than orbitals pointing directly at the anions, which
experience maximum repulsion.
the split in d energy levels (
Δ
oct
) varies with the identity
of the cation and the crystal site. For many transition met-
als in minerals, the energy difference between the d levels
corresponds to the photon energy of visible light. Such
ions are therefore capable of absorbing strongly certain
wavelengths in the visible spectrum, by promoting elec-
trons from the lower level to a vacancy in the upper level
(cf. Figure 6.4). this
crystal field splitting
is the cause of
the strong colours of minerals like malachite and azurite
(Cu - see front cover) or olivine (Fe).
the presence of d-electrons effectively causes a transi-
tion metal ion to deviate significantly from the spherical
shape assumed in Chapter 7. this affects the ease with
which such an ion can be accommodated in a crystal site.
Consider the case of nickel, an important trace element in
basalts: Ni
2+
in a basalt melt crystallizing olivine exhibits
an unexpectedly strong preference for the octahedral Mg
site in olivine, to such an extent that crystallization of
olivine rapidly depletes the Ni content of the melt. the
reason is because the olivine site more readily accommo-
dates the d-orbital geometry of Ni
2+
than do the available
sites in the melt.
(a)
(b)
Co-ordination
polyhedron
cut in half
z
z
Surrounding
anions in
octahedra
grouping
y
x
d
z
2
d
x
2
-y
2
y
3d
Δ
oct
Mean
energy of
3d orbitals in
octahedrally
coordinated cation
d
xy
d
yz
d
zx
3d
d
yz
orbital
in cation
3d energies
split by
octahedral
field
Energy of 3d
orbitals n
isolated cation
Electron
energy
(c)
Figure 9.10.1
Crystal-field splitting of d-orbital energy levels in a transition metal cation in octahedral co-ordination.













































Search WWH ::

Custom Search